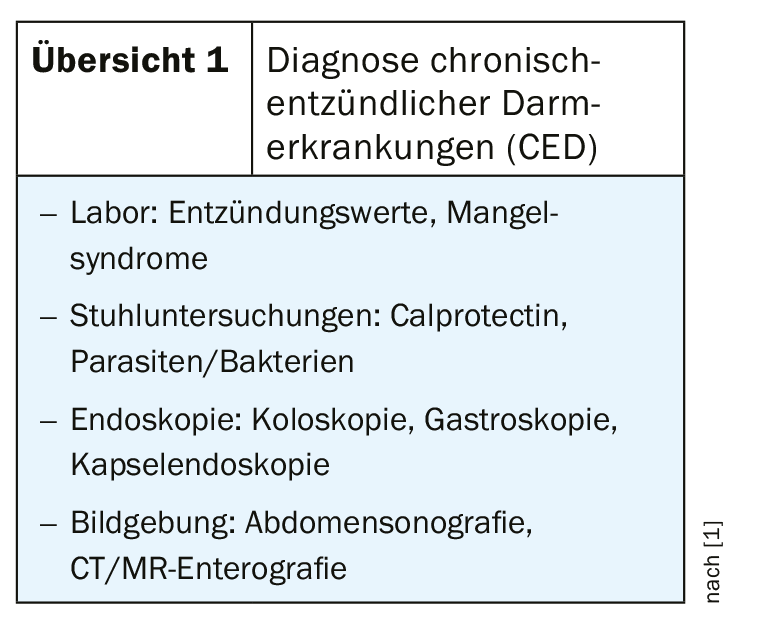
Empirical findings show that calprotectin is the most important laboratory parameter for diagnosis as well as for monitoring the disease and therapy adjustments during its course. The treatment goal of modern therapies is not only freedom from symptoms, but the healing of the intestinal mucosa.
“Crohn’s disease and ulcerative colitis are not only intestinal diseases,” emphasized PD Dr. med. Emanuel Burri, Cantonal Hospital Baselland, on the occasion of the FOMF Update Refresher in Basel [1]. The clinical manifestations of inflammatory bowel disease (IBD) are not limited to the intestine; extraintestinal symptoms are also common. In addition to genetic determinants, interactions of environmental factors with the immune system influence the pathomechanism of this multifactorial disorder. As we know today, intestinal microbiota also play a major role in this process. Differentiation from irritable bowel syndrome is difficult because there is a large overlap with respect to ROM criteria. 40% of all patients with IBD also fulfill the criteria of irritable bowel syndrome [1]. Measurement of calprotectin is an important differential diagnostic parameter with high specificity and sensitivity, the speaker explained. Several meta-analyses demonstrated that high calprotectin levels were associated with inflammatory changes [2,3]. Elevated fecal calprotectin levels, along with weight loss, are among the most important warning symptoms of IBD.
Important prognostic indicator
“Calprotectin shows very well whether there is inflammation in the intestine or not. Not only in diagnostics, but also in the course of treatment,” explains the speaker [1]. Whereas in the past the focus was on symptomatic treatment, nowadays the therapeutic goal is to normalize the intestinal mucosa. Since IBD is a chronic disease, monitoring of the long-term course is central. An increase in calprotectin is an important marker of inflammation in the intestine before symptoms occur. Thus, a calprotectin level >300 (μg/g) during two consecutive months proved to be a prognostic indicator of future recurrence [1,4]. It has been empirically demonstrated that early therapeutic intervention based on measurement of elevated calprotectin levels leads to better healing of the intestinal mucosa after one year compared to starting treatment only when symptoms appear [5]. In the corresponding study, 240 subjects with endoscopically and clinically active Crohn’s disease treated with steroids were randomly assigned to the condition conventional monitoring vs. close-meshed follow-up measurement (CRP, calprotectin) [5].
“Leveraging “Windows of Opportunity
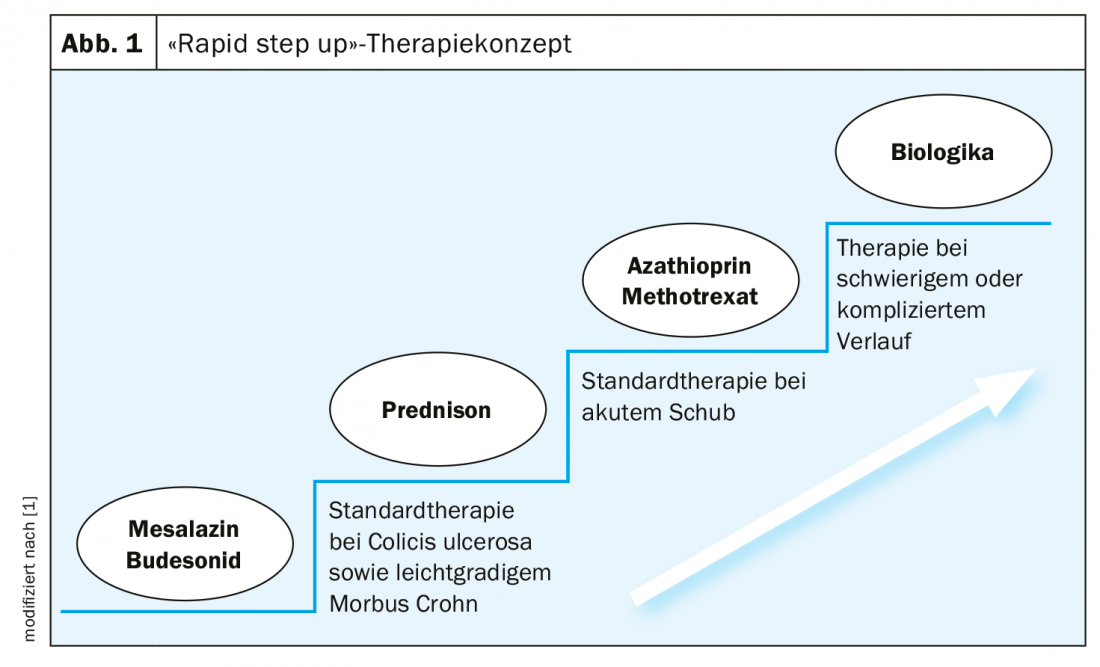
“The assumption today is that if the diagnosis is made very late, structural damage to the intestine has already occurred,” Dr. Burri explains. Therefore, the motto nowadays – as with other chronic inflammatory diseases – is to use effective drugs as early as possible to prevent irreversible damage. In the treatment of IBD, the concept of “rapid step up” therapy is now considered pioneering in Europe (Fig. 1). The market approval of biologics has revolutionized treatment options. The three main targets of these antibody therapies are: Inhibition of inflammatory mediators, inhibition of signal transduction, blocking of inflammatory cells. Janus kinase inhibitors (JAK inhibitors) are a new therapeutic option. These influence the inflammatory cascade by attacking kinases and thus neutralizing several cytokines at the same time. JAK inhibitors offer several advantages: Administration in tablet form, rapid onset of action, no antibody formation against the drug. Unlike biologics, JAK inhibitors intercept cytokine signals intracellularly rather than in the extracellular space. One agent approved in Switzerland for the indication ulcerative colitis is Xeljanz® (tofacitinib) [6].
Source: FOMF Basel
Literature:
- Burri E: Chronic inflammatory bowel disease. Slide presentation, PD Emanuel Burri, MD, FOMF Update Refresher, Basel, Jan. 29, 2020.
- van Rheenen PF, van de Vijver E, Fidler V: Fecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010; 341:c3369. doi: 10.1136/bmj.c3369.
- Lin JF, et al: Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis 2014; 20(8): 1407-1415.
- De Vos M, et al: Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Disease 2013; 19(10): 2111-2117.
- Colombel JF, et al: Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018; 390(10114): 2779-2789.
- Xeljanz®: Swiss Drug Compendium, www.compendium.ch
HAUSARZT PRAXIS 2020; 15(4): 22