For the past year, patients with multiple sclerosis in Switzerland have been able to be prescribed Sativex®, a spray containing delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), without prior special authorization. THC has muscle relaxant and psychoactive effects, while CBD has analgesic, anticonvulsant, neuroprotective, and anxiolytic effects, among others. Studies show a moderate effect in MS-related spasticity. Approximately 50% of treated patients are responders (improvement in spasticity of at least 20% within four weeks). In responders, additional significant improvement in spasticity was achieved in studies over twelve additional weeks of therapy. Experience to date shows that treated patients usually require lower doses than in studies and that there is no dose escalation with long-term use.
“From Pariah to Prescription” was the title of a review paper published a few years ago on the potential medical uses of cannabis [1]. What seemed utopian and provocative at the time has now become reality. For more than a year, physicians throughout Switzerland have been allowed to prescribe Sativex®, a THC-containing spray, to their patients with multiple sclerosis (MS) on a simple narcotic prescription and without prior special authorization from the FOPH. And the state and the scientific community have even given their blessing to this practice, especially since a comprehensive meta-analysis recently published and co-financed by the FOPH concluded that the muscle-relaxing and pain-relieving effects of THC in MS were well established [2].
MS sufferers have long known that cannabis helps
A few years ago, when it was still illegal in many parts of the world to use cannabis even for medical purposes, it was estimated that 15% of all MS patients regularly used the drug hemp (cannabis or marijuana), which was actually prohibited. The firm belief that cannabis could relieve their symptoms of stress, sleep disturbances, muscle spasms, and pain more effectively than conventional medications enticed patients to act in violation of the law. Had these patients really found “the most valuable medicine we possess,” as Dr. J. Russell Reynolds, Queen Victoria’s personal physician put it in the Lancet in 1890? Or had these patients merely become the bona fide victims of unscrupulous hemp dealers?
Why an old Panacea was banished
Cannabis has been known as a painkiller for more than 4000 years and belongs to the group of herbal drugs that, like coca and opium, are still used today. The plant was introduced into European medicine from India in 1842 to relieve pain, muscle spasms, spasms in tetanus, rheumatism, and epilepsy [3]. As Tinctura Cannabis, it was also sold freely in Swiss pharmacies until the 20th century. However, due to quality control problems and political pressures in a world of increasing drug abuse, cannabis was banned from modern Western pharmacopoeias in 1961 when the United Nations decided that cannabis had no medicinal or scientific effect. No wonder – no one knew then that the human body has its own endocannabinoid system with analgesic properties!
Proven therapeutic properties of cannabinoids
Nabiximols, which is marketed as a sublingual spray under the trade name Sativex®, acts precisely in this endocannabinoid system. This alcoholic cannabis extract contains the two main cannabinoids of the hemp plant, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a 1:1 ratio. One stroke contains 2.7 mg THC and 2.5 mg CBD. THC and CBD influence the cannabinoid receptors CB1 and CB2 and exert different, sometimes opposite effects on them. While THC has muscle relaxant and psychoactive effects, among others, CBD has no psychoactive properties (it is therefore not a narcotic like THC), but has analgesic, anticonvulsant, neuroprotective and anxiolytic effects. This mixture has proven to be effective, as CBD is able to attenuate the psychoactive and addictive potential of THC.
CB1 receptors are present throughout the central and peripheral nervous system and interact with numerous neurotransmitters and neuromodulators. Specifically, CB1 receptors have been shown to influence the release of acetylcholine, dopamine, GABA, etc. via retrograde inhibition. The antispastic effects are mainly due to the modulation of the descending inhibitory systems of the spinal cord [4]. CB1 receptors are also found on pain pathways in the brain and spinal cord and are thought to be involved in cannabinoid-induced analgesia. Small, non-psychoactive doses of THC are thought to be sufficient to synergistically exert analgesic effects when combined with opiates. Opiates and cannabinoids combine well, especially since they do not occupy the same receptors. Cannabis suppresses opiate-induced nausea and emesis and leads to effect enhancement, so that the opiate dose can be lowered.
In the guidelines published by the American Academy of Neurology, in addition to the antispasmodic and analgesic properties of cannabinoids, their sedative effect on the overactive bladder is also mentioned [5]. In contrast, no evidence was found that THC attenuates MS-related tremor. Unfortunately, the neuroprotective potential of cannabinoids, which has been repeatedly demonstrated in animal studies, could not be transferred to humans [6].
The spray is effective, but only moderate
In the aforementioned meta-analysis on the medical use of cannabinoids, all randomized controlled trials conducted to date were evaluated according to the GRADE principle (Grading of Recommendations Assessment, Development and Evaluation). Fourteen studies were available on spasticity, including 11 in MS (n=2138) and three in paraplegia (n=142). All studies had placebo control groups.
Overall, the studies show a benefit for nabiximols in MS-related spasticity. In the three studies testing global change on a visual analog scale (VAS), the odds ratio was 1.44 (44% improvement, 95% CI: 1.07-1.94). Furthermore, it is mentioned that nabiximols improves sleep quality more significantly than placebo. Relative to the follow-up period of 3-15 weeks, the GRADE assessment of this large analysis was “moderate evidence” for an effect on MS-associated spasticity as measured by the Ashworth Spasticity Scale or walking speed. For the more stringent outcomes “50% reduction in spasticity at 6-14 weeks follow-up” and “overall impression,” the evidence is judged to be “low grade present.”
The spray helps better with responders
Not all patients respond equally well to nabiximols. For example, one study specifically tested nabiximols in patients who were identified as responders in a preliminary phase. Responders are patients who improve spasticity by at least 20% as measured by a VAS after four weeks of use. In the responders, which included approximately 50% of the patients to be treated, a further significant improvement in spasticity was achieved during the twelve weeks of follow-up therapy [7]. Observations of use over one year confirmed a sustained effect in these patients
[8].
Dispensing only to people with MS
Nabiximols is approved by Swissmedic for symptom improvement in patients with moderate to severe spasticity due to MS who have not responded adequately to other antispasticity drug therapy and who clinically demonstrate significant improvement in spasticity-associated symptoms during the treatment trial (typically four weeks). Here, the subjective impression of the patients is decisive. It is also important when assessing effectiveness to seek the opinion of family members and caregivers.
For the initial treatment of spasticity, muscle relaxants such as tizanidine (Sirdalud®) and baclofen (Lioresal®) are usually used in addition to concomitant physiotherapy. However, as a side effect, they cause muscular weakness due to their action, which can interfere with the ability to walk or stand in a similar way to spasticity. This side effect is attributed to nabiximols or cannabinoids rather less. If the drug therapy recommended as initial treatment does not sufficiently improve spasticity, Sativex® can be used as add-on therapy.
A few strokes per day are usually enough
After a treatment trial of approximately four weeks, only responders should continue to receive nabiximols. Gradual dosing is important to minimize side effects. The dose must be found out variably for each patient. In case of dizziness and lightheadedness, the main dose should be applied in the evening. The maximum dose of twelve sprays per day (30 mg THC/d) is rarely reached, based on experience.
Experience to date shows that treated patients usually require lower doses than in studies and that there is no dose escalation with long-term use. Swissmedic estimates the probability of developing a dependency as low. Nevertheless, nabiximols is a narcotic with the appropriate requirements for prescribing. In patients with substance abuse, the indication should be evaluated particularly carefully. Suicidality, pregnancy, and psychiatric illness are contraindications to nabiximols. During use and up to three months after discontinuation, patients should provide contraceptive measures.
Cognitive impairment may occur and driving ability may be impaired, especially immediately after use and at the start of therapy. The patient must be informed of any reduced ability to drive and, if applicable, impaired ability to work.
Although 62 studies are available that have also investigated side effects, no work has yet been done on possible long-term side effects when nabiximols are used for more than one year.
Overall, the tolerability is rated as “good”.
Cannabis tinctures and oily mixtures on the Swiss market
In the mentioned review, different dosage forms of cannabis extract THC were compared [5]. However, preparations for oral administration (cannabis oil and tinctures) made directly from the plant in Swiss pharmacies have not yet been included in any study. Therefore, the efficacy of these preparations cannot be conclusively assessed in comparison. The review found no significant difference in efficacy between oromucosal use in the form of nabiximols spray, inhaled THC in cigarette form, and orally ingested THC in tablet form. Although oromucosal use of nabiximols results in somewhat more favorable pharmacokinetics and more reliable absorption than oral preparations such as THC tablets and cannabis oil resp. -tincture; however, an equivalent efficiency of the orally taken preparations can be assumed.
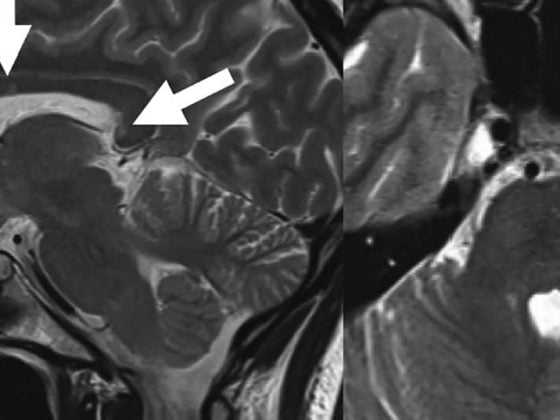
The absorption of THC would be highest by inhalation, but the side effect rate of smoking – which is not yet legalized – is clearly against this form of application. Further, smoked cannabis carries the risk of exacerbating pre-existing cognitive decline due to MS, as demonstrated by an MRI-based study [9].
Sativex® is more expensive but cleaner than hemp from the street
The daily therapy price, calculated for 10 mg THC (4 sprays/d), is CHF 8 for nabiximols (Sativex®). The costs for other cannabis preparations that can be prescribed in Switzerland are in some cases significantly higher (dronabinol solution: 10 mg = 17 CHF; cannabis tincture: 10 mg = 10 CHF, cannabis oil: 10 mg = 16 CHF). Since neither the cannabis preparations mentioned nor Sativex® appear on the list of medicines with tariff, these substances are not covered by the mandatory health insurance.
In practice, it has proven successful to let the patient pay for the first package himself and to apply for a – usually successful – reimbursement from the health insurance company if the substance works. The author has had good experience with this [10].
Further information:
Swiss Working Group on Cannabinoids in Medicine (SACM), www.stcm.ch
Literature:
- Russo E: Introduction: Cannabis: From Pariah to Prescription. Journal of Cannabis Therapeutics 2004; 4(3): 1-29.
- Whiting PF, et al: Cannabinoids for Medical use. A systematic review and meta-analysis. J Amer Med Ass 2015; 313: 2456-2473.
- O’Shaughnessy WB: On the preparations of the Indian Hemp, or Gunjah, (Cannabis Indica): their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci 1843; 123: 363-369.
- Pryce G, Baker D: Potential Control of Multiple Sclerosis by Cannabis and the Endocannabinoid System. CNS & Neurological Disorders – Drug Targets 2012; 11: 624-641.
- Koppel BS, et al: Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014; 82: 1556-1563.
- Zajicek J, et al: Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol 2013; 12: 857-865.
- Novotna A, et al: A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol; 2011; 18(9): 1122-1131.
- Flachenecker P, et al: Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannbidiol oromucosal spray) in clinical practice. European Neurology 2014; 72: 95-102.
- Pavisian B, et al: Effects of cannabis on cognition in patients with MS: a psychometric and MRI study. Neurology 2014; 82: 1879-1887.
- Vaney C: Cannabinoids in MS therapy. Swiss Med For 2016, in press.
InFo NEUROLOGY & PSYCHIATRY 2016; 14(1): 8-10.