Autologous stem cell transplantation is highly valued in the treatment of multiple myeloma. In recent decades, the use of allogeneic blood stem cell transplantation has also been tested, but despite lower recurrence rates, it is now used much less frequently. With CAR-T cells, there is also a new player that could revolutionize the treatment of multiple myeloma.
More than half of all autologous blood stem cell transplants are now performed for multiple myeloma, and the trend is increasing. And this despite the fact that several potent new active ingredients have come onto the market in recent years. The situation is different for allogeneic stem cell transplants: The proportion of multiple myeloma is vanishingly small at about 2% – and the trend continues to decline [1]. Prof. Nicolaus Kröger, MD, Director of the Clinic for Stem Cell Transplantation at the University Medical Center Hamburg-Eppendorf (UKE), explored these questions at the annual meeting of the German, Austrian and Swiss Societies for Hematology and Medical Oncology in Berlin (D).
Allogeneic stem cell transplantation: Two sides of the coin
With a significantly lower recurrence rate, which has been proven in several large studies, the main advantage of allogeneic stem cell transplantation compared to the autologous variant is obvious – especially since the risk of recurrence is still a relevant problem in the treatment of this disease today. After ten years, the relapse rate is 51% for primary allogeneic transplantation and 57% for autologous-allogeneic tandem transplantation. These numbers are small compared with those of autologous tandem transplantation at 74% and simple autologous transplantation at 80%. Thus, hematopoietic stem cell transplantation with donor cells is potentially the greatest chance for a cure for multiple myeloma. But why is this being used with increasing restraint? The reason is the other side of the coin. The immune effect of the donor T cells, which on the one hand leads to the beneficial graft versus myeloma effectand thus presumably to a lower recurrence rate, is on the other hand responsible for the dreaded graft versus host disease(GvHD). This represents the main cause for the comparatively high therapy-associated mortality of allogeneic stem cell transplantation. Thus, the donor T cells used are highly efficient in combating the disease, but not specific enough to prevent negative effects.
Nevertheless, several studies have shown that the reduced recurrence rate after allogeneic stem cell transplantation also translates into survival benefits – especially when considering long-term data. While long-term survival at 20 years was 20% in an analysis from the US Mayo Clinic, it was about 8% after autologous transplantation and 0% without hematopoietic stem cell transplantation [2]. In particular, the introduction of the tandem approach, in which first autologous and then allogeneic stem cell transplantation is performed with dose-reduced conditioning, resulted in some differentiated studies comparing autologous (tandem) transplantation and allogeneic approaches. Although the treatment-related mortality in these studies was lower than with previous concepts, at about 10-15% it was still significantly higher than that of autologous upfront transplants at about 4%. On the other hand, higher rates of complete remission were observed in almost all corresponding publications using allogeneic procedures. Two studies also showed improvement in disease-free and overall survival with the use of donor cells [3,4]. These apparent benefits, which are most striking over the longer course, were also examined in a meta-analysis to achieve the appropriate statistical power [5]. Costa et al. evaluated data on recurrence rate and non-relapse mortality (NRM) from four prospective studies and found what they expected: While the recurrence rate was lower under autologous-allogeneic tandem transplantation, the NRM risk was significantly lower under autologous tandem transplantation. In addition, PFS (HR 0.85; 95% confidence interval 0.75-0.95, p=0.004) and OS (HR 0.84; 95% CI 0.73-0.97, p=0.02) benefits were also shown by donor cell administration in the meta-analysis.
The largest study comparing autologous and allogeneic hematopoietic stem cell transplantation in the first-line treatment of multiple myeloma originated in the United States and was first published in 2011 [6]. This is the BMT-CTN-0102 study, in which significantly higher therapy-associated mortality was observed under autologous-allogeneic transplantation than under autologous tandem transplantation. While the first analysis did not show a statistically significant difference in overall and progression-free survival (PFS), a 10-year follow-up evaluation published in 2020 reported a trend in favor of allogeneic transplantation with respect to PFS, at least in the high-risk group [7]. However, risk classification was based on old criteria, i.e., without the use of molecular genetic markers. No statistically significant PFS or OS differences were detected in the lower-risk group, even after ten years. A German study reached similar results, showing a clear PFS advantage of autologous-allogeneic tandem transplantation only in a small high-risk subgroup of patients with del(13q)+del(17p) [8]. The bottom line is that high-risk patients in particular seem to benefit from this approach. In this patient group, according to Prof. Kröger, the use of the more dangerous allogeneic stem cell transplantation could be justified, but the study situation is by no means sufficient to assess this question and to identify suitable patients. He also said that the place of consolidation and maintenance therapies would need to be characterized more precisely in the coming years.
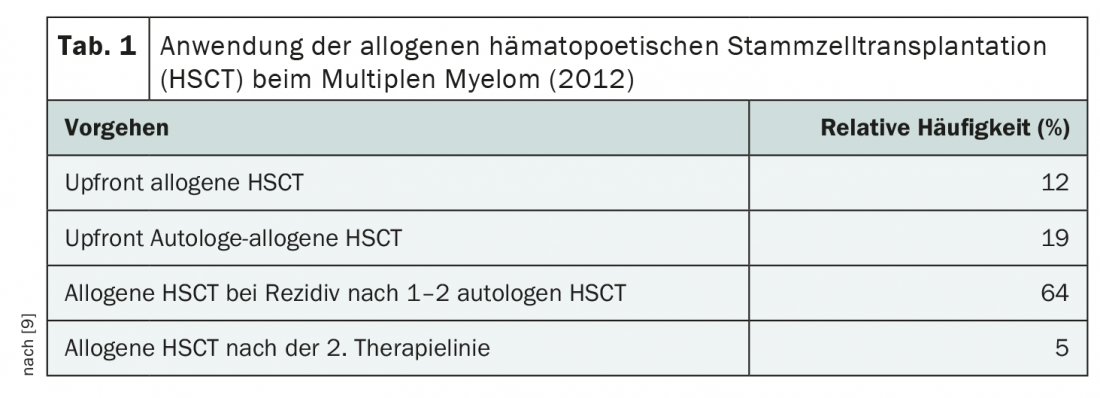
Currently, allogeneic hematopoietic stem cell transplantation is mainly used in later lines of therapy, i.e. in the already relapsed situation, due to the high treatment risk (Tab. 1). Its use has declined significantly in Europe since the early 2000s – despite the fact that its effect is greatest in first-line treatment [9]. According to Prof. Kröger, allogeneic stem cell transplantation should be used at the latest at the first relapse – if at all. After that, the benefit would be too small. The benefit of this second-line therapy is currently being investigated in a large-scale German study. As a potentially curative option, allogeneic stem cell transplantation failed to gain acceptance in the first line of treatment, mainly due to its toxicity profile – which, like efficacy, is via T cell-mediated effects.
CAR-T cells as a solution?
As more specific T cells with at least in more advanced stages already proven efficacy, CAR-T cells have been developed in recent years. Following the approval of axicabtagen ciloleucel and tisagenlecleucel in diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma and B-cell ALL, a first product was recently approved by Swissmedic for the treatment of refractory relapsed multiple myeloma (RRMM) in the fourth line of therapy: Idecabtagen Vicleucel [10]. This is directed against the cell surface protein B-Cell Maturation Antigen (BCMA) and is thus intended to attack malignant cells as specifically as possible. There are other compounds in the pipeline with the same target, such as ciltacabtagene autoleucel, which is currently going through the EMA approval process. While high remission rates have been shown in the various studies, even with extramedullary disease, the recurrence rates and toxicities observed to date are unfortunately not insignificant either. In advanced stages, nota bene. Currently, due to the early stage of development and use in late-stage disease, no comparison with hematopoietic stem cell transplantation is possible. However, CAR-T cells are certainly a promising option on the horizon, according to Prof. Kröger.
Source: Lecture “Auto vs Allo HCT vs CAR-T cell therapy in myeloma” at the scientific symposium “New developments in allogeneic hematopoietic stem cell transplantation”. Nicolaus Kröger, Annual Meeting of the German, Austrian and Swiss Societies of Hematology and Medical Oncology, Oct. 03, 2021, Berlin (D).
Literature:
- Passweg JR, et al: Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021; 56(7): 1651-1664.
- Mir MA, et al: Trends and outcomes in allogeneic hematopoietic stem cell transplant for multiple myeloma at Mayo Clinic. Clin Lymphoma Myeloma Leuk. 2015; 15(6): 349-357.
- Bruno B, et al: A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007; 356(11): 1110-1120.
- Björkstrand B, et al: Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011; 29(22): 3016-3022.
- Costa LJ, et al: Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transplant. 2020; 55(9): 1810-1816.
- Krishnan A, et al: Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011; 12(13): 1195-1203.
- Giralt S, et al: Tandem Autologous versus Autologous-Allogeneic Hematopoietic Stem Cell Transplant for Patients with Multiple Myeloma: Long-Term Follow-Up Results from the Blood and Marrow Transplant Clinical Trials Network 0102 Trial. Biol Blood Marrow Transplant. 2020; 26(4): 798-804.
- Knop S, et al: Allogeneic transplantation in multiple myeloma: long-term follow-up and cytogenetic subgroup analysis. Leukemia. 2019; 33(11): 2710-2719.
- Sobh M, et al: Allogeneic hematopoietic cell transplantation for multiple myeloma in Europe: trends and outcomes over 25 years. A study by the EBMT Chronic Malignancies Working Party. Leukemia. 2016; 30(10): 2047-2054.
- Swissmedic drug information: www.swissmedicinfo.ch (last accessed 04.11.2021).
InFo ONcOLOGy & heMATOLOGy