For more than a decade, biomarkers in cerebrospinal fluid and more recently in blood have revolutionized the diagnosis of Alzheimer’s disease, allowing earlier and more accurate detection of the disease. The 2024 guidelines, with their integrative clinical-biological approach, offer a more complete procedure for the diagnosis and personalized management of the disease, with specific pathways for each stage of the disease.
You can take the CME test on our learning platform after reviewing the recommended materials. Please click on the following button:
Alzheimer’s disease (AD) is the leading cause of dementia worldwide, affecting more than 50 million people. This progressive neurodegenerative disease is characterized by impaired memory, coding and cognitive function associated with the accumulation of amyloid plaques and neurofibrillary tangles in the brain. Historically, diagnosis was mainly based on the observation of clinical symptoms, which were often confirmed post mortem by analysis of the brain. However, with the advent of modern technologies such as neuroimaging and biofluid analysis, AD was gradually redefined as a biological pathology [1]. The introduction of biomarkers that can be detected in body fluids such as cerebrospinal fluid and, more recently, blood, has significantly changed the way the disease is diagnosed, allowing earlier and earlier detection of the disease in research studies. In 2018, the National Institute of Aging (NIA) and the Alzheimer’s Association (AA) introduced the A/T/N (amyloid/tau/neurodegeneration) framework, which enables the diagnosis of AD pathophysiology based on specific biomarkers [2]. This framework has changed the criteria for AD and its diagnosis. This article explores the transition from clinical interpretation of AD to clinical-biological validation, with particular focus on the most promising biomarkers detected in CSF and blood, and the new 2024 guidelines, which include an additional clinical-biological approach for early diagnosis [3].
Biomarkers in the cerebrospinal fluid (CSF)
The cerebrospinal fluid is a biological fluid that is of crucial importance for the investigation of biomarkers of AD. It directly reflects the pathological changes in the brain. The most important biomarkers measured in CSF include amyloid-β 42 (Aβ42), total tau protein (t-tau) and phosphorylated tau protein (p-tau).
Amyloid-β 42 (Aβ42) and theAβ42/Aβ40 ratio: Amyloid-β 42 (Aβ42) is a key protein that forms amyloid plaques in the brain of patients with AD. A decrease in the concentration of Aβ42 in CSF is observed due to its accumulation in the form of plaques. This decrease is an early marker of amyloid pathology. The Aβ42/Aβ40 ratio is an indicator used to improve the accuracy of diagnosis [4,5]. While Aβ42 decreases in the CSF due to plaque formation, the levels of Aβ40, a less pathology-specific form of amyloid protein, remain stable. The Aβ42/Aβ40 ratio enables the correction of individual fluctuations in amyloid β levels and improves the sensitivity and specificity of the diagnosis of AD. A low ratio indicates significant amyloid accumulation and is a reliable indicator of the disease.
The tau protein and its hyperphosphorylation: Total tau (t-tau) in the cerebrospinal fluid is a general marker for neurodegeneration. The tau protein is involved in stabilizing the microtubules of neurons under normal conditions. In AD, however, high concentrations of total tau in the CSF indicate neuronal degeneration. Although t-tau is an indicator of neurodegeneration [6], it is not specific to AD and may also be elevated in other neurodegenerative diseases or after acute brain injury. Phosphorylated tau (p-tau) is a modified form of tau protein in which phosphate groups are added at specific sites, promoting the formation of neurofibrillary tangles, a key feature of AD. The main forms of p-tau measured in CSF are p-tau181, p-tau217 and p-tau231. These biomarkers are more specific for AD than total tau.
- P-tau181 is one of the first phosphorylated tau biomarkers identified for Alzheimer’s disease (AD). Its elevation in CSF reflects the pathological changes associated with neurofibrillary tangles, which are a key feature of AD [7]. p-tau181 is a sensitive and relatively specific biomarker for AD, allowing this disease to be differentiated from other neurodegenerative disorders. Although it is predominantly associated with AD, it may also be slightly elevated in other forms of dementia.
- P-tau217 is more closely associated with AD pathology than p-tau181. Recent studies have shown that p-tau217 correlates better with the formation of amyloid plaques and tau tangles [8,9]. Due to its higher sensitivity and specificity, p-tau217 could gradually replace p-tau181 as a reference biomarker for early diagnosis, especially in the preclinical and mild stages of the disease.
- P-tau231 is a biomarker that is still in the study phase but could provide additional information about the middle stages of AD. Its main use could be in monitoring the transition from mild cognitive impairment (MCI) to dementia [10,11]. Although promising, p-tau231 still requires further research to validate its specificity and potential role in clinical practice.
- MTBR-Tau243: MTBR-Tau243 is a specific and promising biomarker for the detection of tau pathology in Alzheimer’s disease (AD). This biomarker is characterized by the fact that it specifically reflects the insoluble tau aggregates that are strongly associated with the clinical symptoms of AD, in particular cognitive decline. Recent studies have shown that MTBR-Tau243, measured in CSF, is the marker that correlates most strongly with tau PET imaging, outperforming other forms of phosphorylated tau such as p-tau181 and p-tau217. In addition, it shows a significant increase in advanced stages of the disease, making it a relevant indicator for monitoring the progression of tau pathology [12].
In addition to these main biomarkers, other forms such as p-tau205 and p-tau396/404 are gradually being researched [13]. Although they have not yet acquired the same importance as p-tau181 or p-tau217, they could provide additional information about the advanced stages of neurodegeneration. For example, p-tau396/404 is often associated with more severe stages of the disease and could help track disease progression in clinical trials. The combination of t-tau and p-tau may improve the diagnosis of Alzheimer’s disease [14]. While t-tau is indicative of general neuronal degeneration, p-tau is more specific to Alzheimer’s pathology. A high t-tau/Aβ42 ratio is also used as an accurate indicator, with an increase in t-tau combined with a decrease in Aβ42 reflecting advanced neurodegeneration and amyloid pathology [15].
Technological advances in the detection of proteins in plasma
The detection of phosphorylated tau protein (p-tau) in plasma is one of the most promising new developments in the field of biomarkers for Alzheimer’s disease (AD). In the past, the measurement of p-tau in plasma was limited due to its very low concentration and the presence of other circulating proteins. However, highly sensitive technologies such as SIMOA (used by Quanterix) and automated immunoassays (developed by Roche and Fujirebio) have overcome these obstacles [16–20]. They now enable the precise detection of biomarkers such as p-tau181, p-tau217 and the Aβ42/Aβ40 ratio at very low concentrations. Among these biomarkers, p-tau217 stood out for its ability to better classify amyloid and tau pathology, outperforming other biomarkers such as Aβ42/Aβ40, which are often less sensitive due to peripheral variations. These tests are increasingly being used, not only in research, but also for more accessible and less invasive preclinical diagnosis. Other companies, such as C2N Diagnostics, have improved blood tests for the detection of Aβ42/Aβ40 using mass spectrometry. Although this biomarker has a lower accuracy than p-tau217, it remains crucial for the assessment of cerebral amyloid burden, although its interpretation must be combined with other biomarkers to ensure a reliable clinical assessment. Although these innovations have shown great potential for various biomarkers, they need to be further validated before they can be used in clinical practice. However, with appropriate precautions, these technologies could gradually be integrated into clinical practice and offer promising prospects for early, less invasive diagnosis that may be applicable for mass screening in the future.
Biomarkers in the blood
Over the last five years, blood biomarkers have become increasingly important as a non-invasive alternative to CSF testing. Their ease of use and screening potential make them promising tools for the diagnosis of Alzheimer’s disease (AD). Among the most studied biomarkers are phosphorylated tau proteins (p-tau), neurofilament light chain (NfL), gliofibrillary acidic protein (GFAP) and amyloid biomarkers, such as the Aβ42/Aβ40 ratio in plasma. Although these biomarkers offer interesting perspectives, their performance varies depending on the technology used and the stage of the disease [17].
Amyloid-β in blood: a promising alternative, but with limitations: The detection of amyloid proteins in blood, particularly the Aβ42/Aβ40 ratio, represents a turning point in the non-invasive diagnosis of AD. However, recent comparative studies show that tests based on Aβ42/Aβ40 are less powerful overall than tests based on p-tau217 proteins [16]. Indeed, fluctuations due to peripheral factors unrelated to brain pathology, such as the presence of APP in different tissues, complicate the interpretation of this biomarker. Technologies such as SIMOA and Lumipulse allow accurate measurement of this ratio, but systemic variations may affect its specificity. Although amyloid biomarkers can be useful in assessing cerebral amyloid burden, they often need to be combined with other biomarkers to improve the accuracy of diagnosis.
Phosphorylated tau proteins (p-tau): technological differences and clinical implications: The forms of p-tau measured in blood, such as p-tau181, p-tau217 and sometimes p-tau231, play a crucial role in the early diagnosis of AD. According to recent studies, p-tau217 is one of the most promising biomarkers with better diagnostic accuracy than p-tau181, especially in distinguishing between AD and other forms of dementia.
- P-tau181: Although P-tau181 is one of the first forms to be used for blood diagnosis, it has limited sensitivity, especially in very early stages of the disease [8]. Tests such as those offered by Roche and Quanterix show acceptable performance but are inferior to p-tau217 in terms of correlation with amyloid PET results [21].
- P-tau217: According to recent studies, p-tau217, which is detected using technologies such as C2N and Fujirebio Lumipulse, offers higher sensitivity and specificity for the detection of amyloid pathology, outperforming other biomarkers in comparative tests [22]. The performance of p-tau217 is particularly high in the preclinical stages of the disease, making it an important tool for early detection [21].
Neurofilament light (NfL): a non-specific marker of neurodegeneration: NfL is a non-specific marker of neurodegeneration measured in the blood. Although NfL levels increase in several neurodegenerative diseases, including AD, its performance is lower than that of p-tau. The study shows that tests such as the Roche NeuroToolKit and the Quanterix Neurology 4-Plex can detect NfL with good sensitivity, but with lower specificity compared to p-tau217. Nevertheless, NfL remains an excellent marker to follow the progression of neuronal degeneration and to evaluate neuroprotective treatments.
Glial fibrillary acidic protein (GFAP): a marker of glial inflammation: GFAP reflects glial inflammation associated with astrocyte activation in the brain. The study shows that GFAP is a promising marker of inflammation associated with amyloid pathology, with significant increases observed in amyloid-positive patients. However, like NfL, GFAP is not specific to AD and can also be observed in other neuroinflammatory pathologies. Roche and Quanterix technologies enable GFAP to be measured with high accuracy, and the combination with p-tau improves clinical evaluation [25].
Note: The APOE gene, in particular the ε4 allele, is the most important genetic risk factor for AD. Carriers of the ε4 allele have an increased risk of developing the disease [26,27]. This allele is also associated with higher levels of p-tau and amyloid plaques, which increases the likelihood of rapid disease progression. In addition, carriers of APOE ε4 often have elevated GFAP levels, indicating more pronounced neuroinflammation, suggesting that this allele contributes not only to amyloid accumulation but also to the inflammation associated with AD. Consideration of genetics may also serve as a clue for interpreting the results of a plasma analysis in a symptomatic patient.
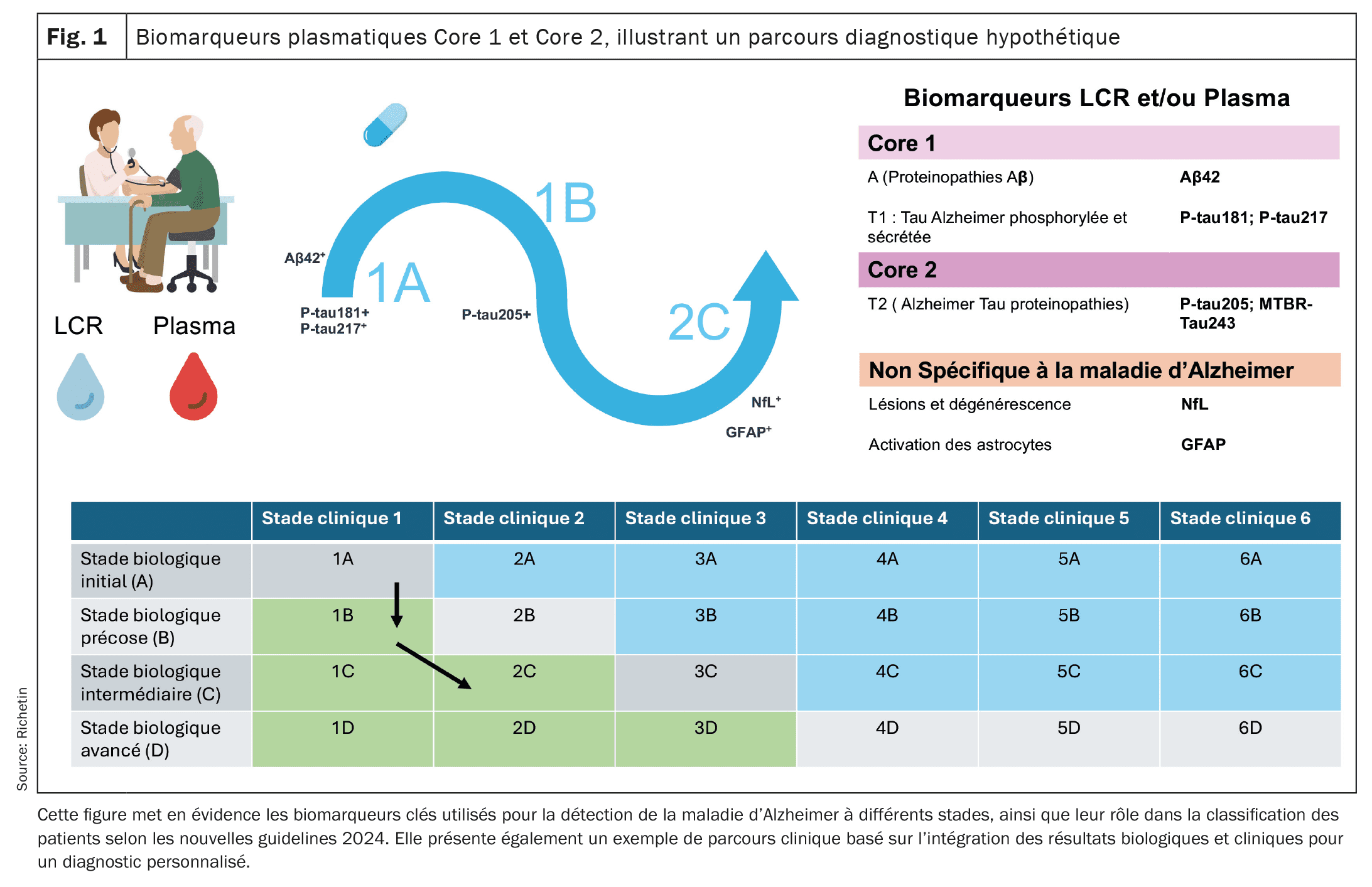
| A new concept of diagnostic pathways is therefore emerging and is likely to be refined over the next few years. So far, the pathways classify patients according to Core 1 and Core 2 biomarker abnormalities and the presence or absence of clinical symptoms to tailor diagnosis and follow-up. The new guidelines could provide a more accurate framework for diagnostic assessment and monitoring the progression of AD in a more personalized way by incorporating plasma and CSF biomarkers as well as clinical assessments. They will also facilitate appropriate treatment at each stage of the disease, from the preclinical phase to the advanced stages of neurodegeneration. |
From research to diagnosis?
In recent decades, advances in the study of biomarkers for Alzheimer’s disease have enabled a decisive transition to increasingly accessible clinical application and precision diagnostics. Initial work focused on the identification of amyloid pathology and tau proteinopathy in cerebrospinal fluid (CSF) and led to the development of tests that can detect these abnormalities with high accuracy. These biomarkers, initially used in research, have made it possible to confirm the presence of pathophysiological alterations prior to symptoms. However, access to these tests was limited to specialized research centers due to the invasive and costly techniques involved. With the introduction of plasma biomarkers, particularly p-tau forms and the Aβ42/Aβ40 ratio, research has taken a decisive step towards broader clinical application. The ability to detect these biomarkers in blood using highly sensitive technologies has paved the way for non-invasive screening that is more feasible in the clinic. These innovations may make it possible to easily confirm the pathophysiology of AD in patients with suggestive clinics and to identify asymptomatic individuals at risk of developing AD even before cognitive symptoms appear. However, challenges remain regarding standardization of tests, their variability (circadian variation, renal function, vascular), their availability outside major centers, and reimbursement, especially for plasma testing. The gradual integration of biomarkers into daily treatment routines, combined with imaging technologies such as amyloid PET and tau PET, will allow not only earlier diagnosis but also better assessment of disease progression. This transition from research to clinical application represents a major advance for personalized medicine and could enable strategies for secondary or tertiary prevention depending on the stage of the disease.
Towards a more integrated clinical-biological approach
In the summer of 2024, the National Institute of Aging (NIA) and the Alzheimer’s Association proposed new guidelines based on an approach that combines clinical and biological assessments to improve the diagnosis and monitoring of Alzheimer’s disease (AD) progression [16]. These guidelines are based on two categories of biomarkers: Core 1 and Core 2, and on several diagnostic pathways (1A, 1B, etc.) that make it possible to classify patients according to the progression of their disease. Core 1 biomarkers directly measure amyloid and tau pathology. They include indicators such as the beta-amyloid ratio Aβ42/Aβ40 and the forms of phosphorylated tau (p-tau181, p-tau217). These abnormalities appear in the early stages of the disease, often in correlation with amyloid PET imaging results, facilitating early diagnosis even before clinical symptoms appear. Plasma biomarkers, particularly p-tau217 and p-tau181, have been shown to be useful in detecting these abnormalities in plasma, allowing for broader and less invasive screening. However, the interpretation of these results must be combined with a thorough clinical evaluation. Core-2 biomarkers reflect processes of neurodegeneration and advanced tau proteinopathy, such as MTBR-tau243. They become abnormal in later stages of AD, which is directly related to the onset of cognitive symptoms. Biomarkers such as neurofilament light chain (NfL) and gliofibrillary acidic protein (GFAP), detected in blood and cerebrospinal fluid (CSF), provide valuable information about neuronal degeneration and inflammation and play a crucial role in assessing disease progression and adjusting treatment strategies.
Proposal of a hypothetical scenario for the diagnostic and therapeutic pathway of a patient with Alzheimer’s disease
Step 1: Screening in a memory center (course 1A): Mr. K., age 62, comes in for a routine checkup with no apparent cognitive symptoms. Given his family history of Alzheimer’s disease, he would be referred for screening at a memory center. A blood test might reveal abnormally high p-tau217 levels and a reduced Aβ42/Aβ40 ratio, possible indicators of amyloid pathology. These findings would be confirmed by cerebrospinal fluid (CSF) analysis showing abnormal accumulation of amyloid and tau proteins. At this stage, Mr. K would have no clinical symptoms, but his Core 1 biomarkers (p-tau and Aβ) could indicate Alzheimer’s pathology in the preclinical phase (pathway 1A). Given the early detection of this amyloid pathology, it would be conceivable to offer an anti-amyloid therapy such as lecanemab or donanemab to slow down the formation of amyloid plaques in the brain. These therapies, administered at a preclinical stage, could aim to delay progression to more severe cognitive symptoms.
Step 2: Onset of mild cognitive impairment (course 1B): Two years later, Mr. K may begin to notice mild memory disturbances such as forgetting appointments. A clinical re-evaluation would then be performed, including a new set of blood tests and a lumbar puncture. The results could show persistent abnormalities in core-1 biomarkers, which would confirm the continuity of amyloid and tau pathology. Neuropsychological testing might reveal mild cognitive deterioration. At this stage, Mr. K would enter progression 1B, which is characterized by the presence of Mild Cognitive Impairment ( MCI) associated with abnormal biomarkers. This development may warrant more intensive monitoring and possible adjustment of anti-amyloid therapy to enhance prevention of more severe symptoms. These therapies would continue to be administered to delay progression to confirmed dementia.
Step 3: Development into advanced neurodegeneration (progression 2C): Five years after initial diagnosis, Mr. K’s symptoms would worsen. He might exhibit a more severe cognitive impairment, affecting his daily activities and marking the transition to advanced dementia. A new set of biomarker analyses would be performed, revealing abnormalities in core-2 biomarkers such as elevation of p-tau205. In addition, biomarkers of neurodegeneration such as NfL (neurofilament light chain) and GFAP (glial fibrillary acidic protein) could indicate active neuroinflammation and destruction of neurons. Tau PET imaging could show extensive accumulation of tau in regions associated with cognition. At this stage, Mr. K would be in treatment pathway 2C, with marked neurodegeneration. The treatment plan would then focus on treating the cognitive and behavioral symptoms, with a combination of symptomatic therapies, individualized care, and increased family support to maintain the patient’s quality of life.
Conclusion: This hypothetical scenario underlines the importance of early detection by biomarkers in blood and cerebrospinal fluid in Alzheimer’s disease. It also shows that the introduction of anti-amyloid therapies could delay the progression of the disease before the onset of symptoms. The continuous monitoring of biomarkers in conjunction with neuropsychological assessments would make it possible to adapt therapeutic measures to biological and clinical evolution, thus ensuring personalized treatment of the patient.
Clinical benefits of early diagnosis
The early diagnosis of Alzheimer’s disease, made possible by biomarkers in cerebrospinal fluid and blood, brings great clinical benefits. It makes it possible to intervene at a stage where treatments, while limited in their ability to reverse the disease, can slow the progression of cognitive symptoms. By detecting the disease at stage 1A, when patients are not yet showing clinical signs, clinicians could introduce preventative treatment strategies to delay the progression to more symptomatic stages. Another important benefit of early detection is that it allows for better planning of long-term care. Once pathology has been identified by amyloid or tau biomarkers, patients and their families can benefit from better anticipation of disease progression. This includes more informed decisions about care, financial management and the organization of daily life, as well as the establishment of appropriate support networks. This proactive approach can improve the patient’s quality of life by delaying the disabling effects of dementia and promoting personalized care.
Early diagnosis using biomarkers such as p-tau217 and Aβ42/Aβ40 also enables more targeted and patient-specific treatment decisions. Clinicians can determine earlier whether a patient is suitable for clinical trials of new therapies or for disease-modifying treatments aimed at slowing amyloid or tau accumulation. Treating patients in the early stages of the disease can also limit the impact of secondary complications associated with AD progression, such as loss of independence, behavioral disturbances and increased burden on family caregivers. Finally, early diagnosis enables better management of medical resources, especially in countries with limited resources [28]. By more accurately identifying patients who would benefit most from close monitoring or specific interventions, clinicians can adapt their practices and prioritize care according to the severity of the disease. This could also have a positive economic impact by reducing the long-term costs associated with late-stage care of advanced dementia, which often involves hospitalization and more complex care.
Conclusion
Biomarkers in cerebrospinal fluid and blood have revolutionized the diagnosis of Alzheimer’s disease, enabling earlier and more accurate detection of the disease even before clinical symptoms appear. The 2024 guidelines, with their integrative clinical-biological approach, provide a more complete process for the diagnosis and personalized management of the disease with specific pathways for each stage of the disease. However, despite these advances, further efforts are needed to make these technologies more accessible, particularly by expanding their availability outside of specialized centers and addressing cost and reimbursement issues. These initiatives will be critical to improving the management of this disease on a large scale by providing more equitable access to early diagnosis and more appropriate therapeutic interventions and monitoring.
Take-Home-News
- For more than a decade, biomarkers in cerebrospinal fluid and, more recently, in blood have revolutionized the diagnosis of Alzheimer’s disease, enabling earlier and more accurate detection.
- The 2024 guidelines, with their integrative clinical-biological approach, provide a more complete process for the diagnosis and personalized management of the disease with specific pathways for each stage of the disease.
- However, despite these advances, further efforts are needed to make these technologies more reliable and accessible. Biomarkers for Alzheimer’s disease in the blood could change the way Alzheimer’s disease is diagnosed early in the next few years and provide a window for intervention before clinical symptoms appear.
- Markers such as p-tau217 and the Aβ42/Aβ40 ratio promise to increase diagnostic accuracy so that therapeutic strategies can be considered to slow disease progression.
- These advances would provide clinicians with more sensitive, less invasive instruments that could potentially be used in routine clinical practice.
- Although there are still challenges in terms of standardization and cost to healthcare systems, the 2024 guidelines would pave the way for personalized management that combines biological and clinical assessments to optimize follow-up care at every stage of the disease.
Literature:
- Huang S, Wang YJ, Guo J: Biofluid Biomarkers of Alzheimer’s Disease: Progress, Problems, and Perspectives (Biofluid Biomarkers of Alzheimer’s Disease: Progress, Problems, and Perspectives). Neuroscience Bulletin 2022 38: 6 38, 677-691 (2022).
- Jack CR, et al: NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia 2018; 14: 535-562.
- Jack CR, et al: Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s and Dementia 2024; 20: 5143-5169.
- Salvadó G, et al: Specific associations between plasma biomarkers and postmortem amyloid plaque and tau tangle loads. EMBO Mol Med 2023; 15.
- Hansson O, Lehmann S, Otto M, et al: Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res Ther 2019; 11: 1-15.
- Holper S, Watson R, Yassi N: Tau as a Biomarker of Neurodegeneration. International Journal of Molecular Sciences 2022; 23: 7307.
- Kurihara M, Komatsu H, Sengoku R: FULL-LENGTH ARTICLE CSF P-Tau181 and Other Biomarkers in Patients With Neuronal Intranuclear Inclusion Disease. Cited as: Neurology® 2023; 100: 1009-1019.
- Janelidze S, et al: Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med (2020), doi: 10.1038/s41591-020-0755-1.
- Janelidze S, et al: Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nature Communications 2020; 11(1): 1-12.
- Ashton NJ, et al: Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol 2021; 141: 709-724.
- Buerger K, et al: CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology 2002; 59: 627-629.
- Horie K, et al: CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nature Medicine 2023; 29(8): 1954-1963.
- Lantero-Rodriguez J, et al: CSF p-tau205: a biomarker of tau pathology in Alzheimer’s disease. Acta Neuropathol 2024; 147: 1-17.
- Olsson B, et al: CSF and blood biodomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016; 15: 673-684.
- Santangelo R, et al: The CSF p-tau181/Aβ42 Ratio Offers a Good Accuracy “In Vivo” in the Differential Diagnosis of Alzheimer’s Dementia. Curr Alzheimer Res 2019; 16: 587-595.
- Schindler SE, et al: Head-to-head comparison of leading blood tests for Alzheimer’s disease pathology. Alzheimer’s & Dementia (2024), doi: 10.1002/ALZ.14315.
- Hampel H, et al: Blood-based biomarkers for Alzheimer’s disease: Current state and future use in a transformed global healthcare landscape. Neuron 2023; 111: 2781-2799.
- Hansson O, et al: The Alzheimer’s Association appropriate use recommendations for blood biod biomarkers in Alzheimer’s disease. Alzheimer’s & Dementia 2022; 18: 2669-2686.
- Hansson O, Blennow K, Zetterberg H, Dage J: Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nat Aging 2023; 3: 506-519.
- Álvarez-Sánchez L, Peña-Bautista C, Baquero M, Cháfer-Pericás C: Novel Ultrasensitive Detection Technologies for the Identification of Early and Minimally Invasive Alzheimer’s Disease Blood Biomarkers. Journal of Alzheimer’s Disease 2022; 86: 1337-1369.
- Blennow K, et al: The potential clinical value of plasma biomarkers in Alzheimer’s disease. Alzheimer’s & Dementia 2023; 19: 5805-5816.
- Ashton NJ, et al: Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer’s Disease Pathology. JAMA Neurol 2024; 81: 255-263.
- Alcolea D, Beeri MS, Rojas JC, et al: Blood Biomarkers in Neurodegenerative Diseases: Implications for the Clinical Neurologist. Neurology 2023; 101: 172-180.
- Palmqvist S, et al: Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer’s Disease vs Other Neurodegenerative Disorders (Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer’s Disease vs Other Neurodegenerative Disorders). JAMA 2020; 324: 772-781.
- Kim KY, Shin KY, Chang KA: GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023: 12.
- Yakoub Y, et al: Longitudinal blood biomarker trajectories in preclinical Alzheimer’s disease. Alzheimers Dement 2023; 19: 5620-5631.
- Stevenson-Hoare J, et al: Plasma biomarkers and genetics in the diagnosis and prediction of Alzheimer’s disease. Brain 2023; 146: 690-699.
- Nwamekang Belinga L, et al: Circulating Biomarkers for Alzheimer’s Disease: Unlocking the Diagnostic Potential in Low- and Middle-Income Countries, Focusing on Africa. Neurodegener Dis 2024; 24: 26-40.
InFo NEUROLOGIE & PSYCHIATRIE 2024; 22(6): 14-19