Lifestyle modification (exercise, diet) and metformin remain the cornerstones in the treatment of diabetes mellitus type 2. Before initiating antidiabetic therapy, it is recommended to set an individual HbA1c target. Advantages of the new oral antidiabetic drugs (OADs) are the low risk of hypoglycemia, weight loss or weight neutrality, and usually good tolerability. The SGLT2 inhibitors induce renal glucose loss and are effective only with preserved renal function. Due to the novel mechanism of action, they can be well combined with other OADs. Based on the efficacy and side effect profile of the new antidiabetic drugs, the aim is to find the best possible individual therapy combination for each patient.
Due to new findings and developments, important new principles have been established in the treatment of type 2 diabetes and have found their way into the current guidelines: An individual HbA1c target in the range of 6.5-8.0% should be set for each patient, taking into account disease duration, concomitant diseases, life expectancy, and personal and social resources. A reduction of macrovascular complications can be achieved if a sustainable optimal diabetes control is achieved from the beginning of the disease (HbA1c 6.5-7.0%). In contrast, lowering HbA1c <7.0% “at any cost” leads to frequent hypoglycemia and an increase in mortality in the presence of pre-existing cardiovascular complications and long disease duration [1]. While the role of metformin as first-line therapy along with lifestyle modification is undisputed, there are numerous combination options to choose from in the second step after the introduction of incretins and gliflozines, which will be discussed in detail below.
News on the “old” oral antidiabetics
Metformin: At or shortly after diagnosis of type 2 diabetes, all patients should receive therapy with metformin if optimal diabetes control cannot be achieved by lifestyle modification and treatment is gastrointestinally tolerated. Metformin is strongly HbA1c-lowering, reduces microvascular and macrovascular complications, delays diabetes progression, does not cause hypoglycemia, supports weight loss, and is cost-effective. Renal insufficiency with an eGFR <30 ml/min is an important contraindication due to the risk of life-threatening lactic acidosis. If eGFR drops to 30-45 ml/min, pre-existing therapy can be continued at a maximum of 1000 mg daily, and initiation of therapy is not recommended. Treatment can be restarted at an eGFR of 45-60 ml/min, with maximum daily dose recommendations of 1000-2000 mg varying [2].
It is crucial to instruct patients to pause therapy for intercurrent conditions (surgeries, infections, heart failure, etc.). If the use of iodine-containing radiographic contrast media is planned, metformin should be stopped 48 hours beforehand at an eGFR <45 ml/min (intravenous administration) or <60 ml/min (intrarterial administration). With documented stable renal function, resumption of therapy is possible 48 hours after contrast administration [3]. As a result of interference with vitamin B12 absorption, vitamin B12 deficiency occurs in 10-15% of patients on metformin and should be sought and substituted if signs are present, especially in peripheral polyneuropathy.
Gliclazide: Of the available sulfonylureas, only gliclazide is recommended because longer-acting preparations, especially glibenclamide, have an increased risk of hypoglycemia and their use may be associated with increased mortality [4]. With only a modest increase in hypoglycemia risk, cardiovascular safety and reduction in the incidence of microvascular complications have been adequately demonstrated with gliclazide [5]. Gliclazide is potent HbA1c-lowering agent, its use associated with a slight increase in weight.
Glitazones: Of the glitazones, only pioglitazone is still available after rosiglitazone was withdrawn from the market in 2010 because increased cardiovascular events were observed with the therapy. The mechanism of action is based on an improvement in insulin sensitivity, resulting in a potent HbA1c-lowering effect without an increased risk of hypoglycemia. Glitazones cause weight gain by increasing subcutaneous adipose tissue and by fluid retention, so they cannot be used in heart failure. In addition, osteoporotic fractures have increased in postmenopausal women in recent studies [6]. The association of pioglitazone use with an increased risk of bladder cancer has been questioned or put into perspective by a recently published study [7]. Nevertheless, with the availability of new substances, pioglitazone has become less important and is now used relatively rarely, at least in Switzerland.
New (oral) antidiabetic agents
Gliflozine: It has been known since the 19th century that phlorizin, a glycoside first isolated from the bark of apple trees in 1835, has a glucosuric effect. In 1987, phlorizin was first used in animal models as an antidiabetic agent and shortly thereafter was recognized as an inhibitor of sodium glucose cotransporters (SGLT) 1 and 2 (sodium glucose cotransporters of the kidney). SGLT2 is mainly found in the proximal tubule of the kidneys, SGLT1 in the proximal tubule and small intestine. Due to poor oral bioavailability, phlorizin did not find its way into the clinic but stimulated the development of new SGLT inhibitors. To date, three relatively selective SGLT2 inhibitors, canagliflozin (Invokana®), dapagliflozin (Forxiga®), and empagliflozin (Jardiance®), are available and their use results in renal glucose loss of 60-90 g/d [8]. These agents lower HbA1c by approximately 0.5-1% compared with placebo, regardless of whether an SGLT2 inhibitor was administered as monotherapy or in combination. A reduction in both fasting and postprandial blood glucose levels was noted. Welcome side effects included systolic blood pressure reduction (2-8 mmHg at six months) and body weight loss (1-3 kg at six months). In the presence of impaired renal function (GFR <45-60 ml/min), the effect of SGLT2 inhibitors decreases rapidly [9].
Genital mycoses occur more frequently with SGLT2 inhibitors (approx. 10%), which is why good intimate hygiene must be observed. If mycosis occurs, we prefer single-agent oral therapy with fluconazole. Further, urinary tract infections are also somewhat clustered. Due to the osmotic diuresis, orthostatic complaints may occur, so that the adjustment of any existing diuretic therapy should be considered at the start of therapy [8].
Long-term data regarding safety and reduction of microvascular and macrovascular endpoints are not yet available. Thus, a slight increase in LDL cholesterol and a decrease in HDL cholesterol of about 4% were observed in various studies. Recently, an incidence of ketoacidosis associated with the use of SGLT2 inhibitors has also been observed in patients with type 2 diabetes. In most cases, a predisposing factor was identifiable- severe illness, decreased food and fluid intake, or a reduction in the insulin dose used. It is also worth mentioning that the ketoacidoses occurred with only slightly elevated blood glucose levels (euglycemic ketoacidosis), which made the diagnosis difficult [10]. SGLT2 inhibitors, like metformin, should be paused during intercurrent illness, surgery, etc.
The gliflozines can be combined with metformin, gliclazide, gliptins, GLP-1 analogues, and insulin. The combination with a gliptin is potent HbA1c-lowering and does not cause hypoglycemia (Fig. 1) [11]. Currently, only dapagliflozin can be combined with the gliptins in Switzerland without prior cost approval. The combination with GLP-1 analogues is also potentially very effective, but has not yet been approved in Switzerland. SGLT2 inhibitors can also be combined with insulin and then often allow a reduction in insulin dose. They are also effective in type 1 diabetes, but are not approved for this indication.
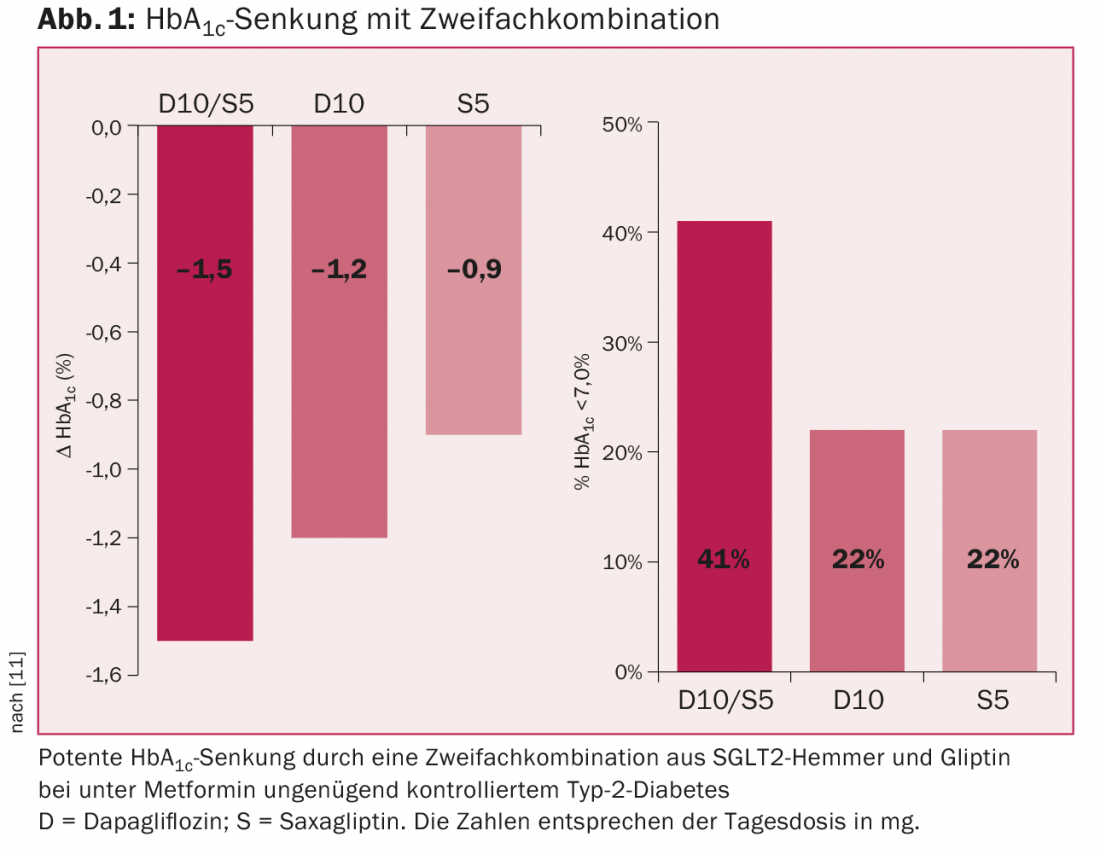
In summary, the use of an SGLT2 inhibitor is particularly useful in younger obese patients with normal renal function, in whom hypoglycemia must also be avoided at all costs (e.g., chauffeurs).
incretins (gliptins and GLP-1 analogs): Gliptins are inhibitors of dipeptidyl peptidase 4 and thus prevent the rapid degradation of incretins, especially GLP-1 (“glucagon like peptide 1”). Enhancement of incretin or GLP-1 action results in, among other things, delay of gastric emptying, activation of β-cells with increased insulin secretion, and inhibition of α-cells (glucagon secretion) and gluconeogenesis in the liver. Gliptins thus improve fasting and postprandial blood glucose levels. HbA1c reduction with monotherapy can be expected in the range of 0.5-1.0% [12]. Gliptins are weight neutral and do not cause hypoglycemia due to glucose-dependent action. In general, gliptins are very well tolerated. After concerns were raised about cardiovascular safety based on prior results (increased hospitalizations due to heart failure with saxagliptin in the Savor-TIMI 53 study), follow-up studies showed no increased heart failure or cardiovascular risk [13–15]. However, long-term data demonstrating a reduction in microvascular and/or macrovascular end points remain unavailable.
In moderately and severely impaired renal function (eGFR <30 ml/min), only linagliptin can be used without dose adjustment. Gliptins can be combined with metformin, gliclazide, SGLT2 inhibitors, and basic insulin. The combination with intensified insulin therapy is not recommended due to the low additional benefit.
Modification of the GLP-1 molecule can also prevent its rapid degradation by dipeptidyl transferase 4. Subcutaneous application of these GLP-1 analogs achieves supraphysiological GLP-1 levels, so the effect of GLP-1 analogs is more potent compared with gliptins. The HbA1c reduction achieved in clinical trials is 1.0-1.8%. The currently available GLP-1 analogues differ mainly in their duration of action: exenatide (Byetta®) and lixisenatide (not available in Switzerland) are short-acting (half-life <5 hours) and primarily lower postprandial blood glucose levels, while the long-acting preparations (half-life >12 Hours: Liraglutide [Victoza®], Exenatide LAR [Bydureon®], Dulaglutide [Trulicity®], and Albiglutide [Eperzan®]) lower fasting blood glucose levels more. All GLP-1 analogues result in weight loss, on average in the range of 1-4 kg, and do not cause hypoglycemia. Gastrointestinal side effects such as nausea, bloating and diarrhea are relatively common and more pronounced with the short-acting preparations, but usually subside after a few weeks. If symptoms persist, treatment with an alternative long-acting GLP-1 analogue may be attempted. Subcutaneous administration is daily (liraglutide) or weekly (exenatide LAR, dulaglutide, albiglutide). GLP-1 analogues can be combined with metformin, gliclazide, and pioglitazone; concomitant prescription of gliptins is futile because of the same target. Only liraglutide can be combined with basic insulin (approval and reimbursement) [16]. GLP-1 analogues have not been studied or approved in significantly impaired renal function (eGFR <30 ml/min) .
The newly available fixed combination (IDegLira, Xultophy®) of a basic insulin (Degludec, Tresiba®) with a GLP-1 analog (liraglutide) is a very potent treatment option with few side effects and is associated with a very low risk of hypoglycemia. In patients already treated with a basic insulin and metformin, a HbA1c reduction of 1.9% was achieved by switching to this therapy. Regular dose titration (once or twice a week) based on morning blood glucose levels is critical for treatment success [17].
Conclusion
The new antidiabetic agents open up new therapeutic options in patients whose diabetes is inadequately controlled on metformin or a two-drug combination. SGLT2 inhibitors in particular can be combined with all blood glucose-lowering drugs due to their special mechanism of action. Very potent HbA1c reduction (>1.5%) can be achieved with combination therapies. None of the new substance groups has yet been shown to lead to a reduction in microvascular and/or macrovascular complications. Long-term studies demonstrate the cardiovascular safety of gliptins.
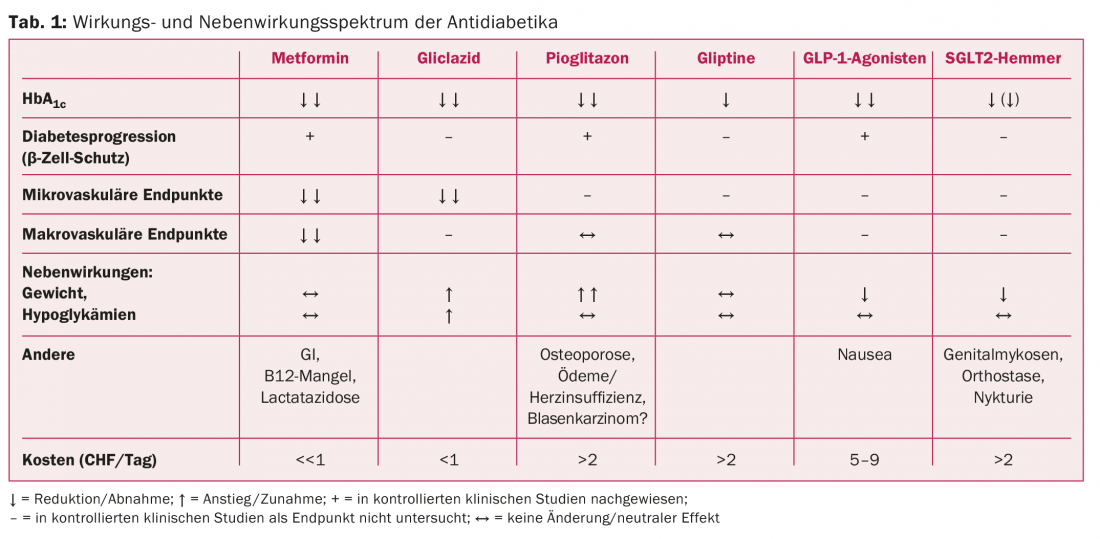
Table 1 shows the spectrum of action and side effects of antidiabetic drugs.
Literature:
- American Diabetes Association, ADA Position Statement 2015: 7. Approaches to Glycemic Treatment. Diabetes Care 2015 Jan 1; 38(Suppl 1): S41-48.
- Inzucchi SE, et al: Metformin in Patients With Type 2 Diabetes and Kidney Disease: A Systematic Review. JAMA 2014 Dec 24; 312(24): 2668.
- Swiss Society of Endocrinology and Diabetology: metformin and iodine-containing roentgen contrast agents [Internet]. Guidelines. 2014 [cited 2015 Jul 29]. Available from: www.sgedssed.ch/fileadmin/files/news/Metformin_und_jodhaltige_Roentgenkontrastmittel.pdf
- Simpson SH, et al: Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol 2015 Jan; 3(1): 43-51.
- ADVANCE Collaborative Group, Patel A, et al: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008 Jun 12; 358(24): 2560-2572.
- Soccio RE, Chen ER, Lazar MA: Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab 2014 Oct 7; 20(4): 573-591.
- Lewis JD, et al: Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA 2015 Jul 21; 314(3): 265-277.
- Tahrani AA, Barnett AH, Bailey CJ: SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol 2013; 1(2): 140-151.
- Vasilakou D, et al: Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013 Aug 20; 159(4): 262-274.
- Peters AL, et al: Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015 Jun 15; dc150843 (Epub ahead of print).
- Rosenstock J, et al: Dual Add-on Therapy in Type 2 Diabetes Poorly Controlled With Metformin Monotherapy: A Randomized Double-Blind Trial of Saxagliptin Plus Dapagliflozin Addition Versus Single Addition of Saxagliptin or Dapagliflozin to Metformin. Diabetes Care 2015 Mar; 38(3): 376-383.
- Karagiannis T, et al: Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012; 344: e1369.
- Scirica BM, et al: Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013 Oct 3; 369(14): 1317-1326.
- Zannad F, et al: Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015 May 23; 385(9982): 2067-2076.
- Green JB, et al: Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015 Jul 16; 373(3): 232-242.
- Meier JJ: GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012 Dec; 8(12): 728-742.
- Buse JB, et al: Contribution of Liraglutide in the Fixed-Ratio Combination of Insulin Degludec and Liraglutide (IDegLira). Diabetes Care 2014 Nov; 37(11): 2926-2933.
CARDIOVASC 2015; 14(4): 4-8











