Drug options in the treatment of lung cancer have developed rapidly in recent years. In addition to classical chemotherapy, immunotherapy and targeted therapy of driver mutations are increasingly coming to the fore. The importance of biopsy and also re-biopsy under therapy has increased enormously.
Drug options in the treatment of lung cancer have developed rapidly in recent years. In addition to classical chemotherapy, immunotherapy and targeted therapy of driver mutations are increasingly coming to the fore. The basic prerequisite for personalized drug therapy is the immunohistochemical and molecular pathological examination of the tumor tissue. Due to these developments, the importance of biopsy and also re-biopsy under therapy has increased enormously.
Due to the favorable risk-benefit ratio, bronchoscopy is the method of choice. The examination must provide a comprehensive clarification of the thoracic situation. This includes central and peripheral tumor manifestations and mediastinal lymph node status.
A mandatory prerequisite for this is the presence of thoracic cross-sectional imaging at least in the form of a contrast-enhanced CT, optimally a combined PET-CT.
Central tumor
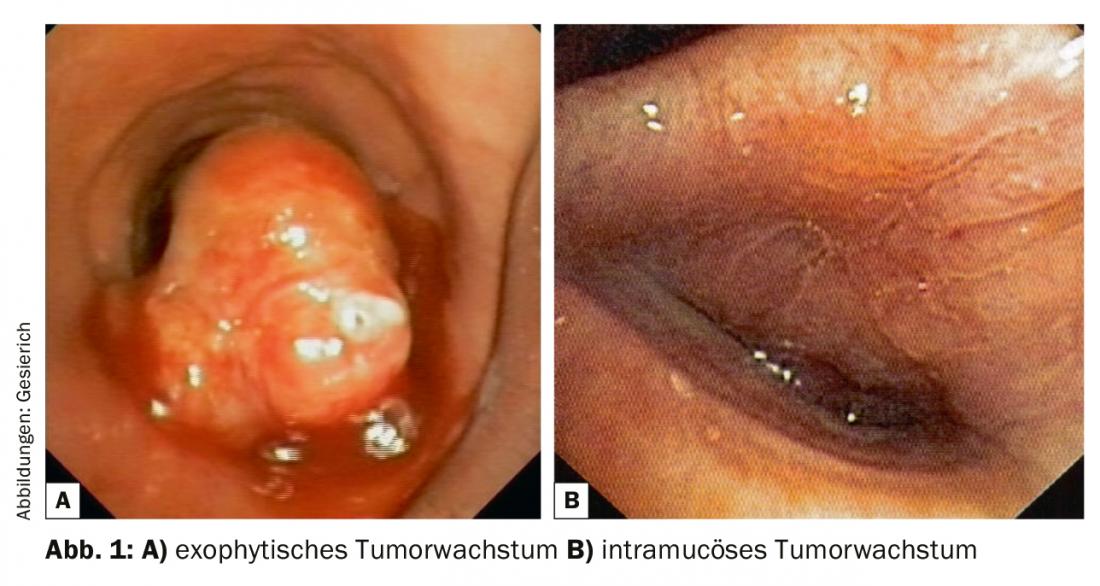
A central tumor involvement in the bronchoscopically visible area must first be described in terms of its localization and extension. The distance to the nearest proximal and distal landmarks (main carina, lobe, segment, and subsegment ostia) and the length of the tumor-affected bronchial segment must be reported. In this context, the bronchologist must be familiar with the thoracic surgical options, including complex bronchoplastic procedures (“cuff resections”). The type of tumor involvement (exophytic, intramucosal, submucosal) should be described. Any resulting airway stenosis (endoluminal-exophytic; extrinsic compression; combination stenoses) must be characterized and quantified.
The next step is biopsy, taking into account the greatly increased tissue requirements of pathology to also perform immunohistochemical and molecular analyses. In the case of exophytic tumor (Fig. 1), forceps biospies are usually sufficient for this purpose. If ablation of tumor exophytes for recanalization is planned at the same time, extraction with the cryoprobe is also an option, providing excellent samples for histopathological processing. This type of material collection also offers advantages in cases of intramucosal tumor growth because of the greater biopsy depth. In cases of submucosal growth, transbronchial needle aspiration (TBNA) is usually required for histologic confirmation. This can be done with conventional, flexible TBNA needles, but endobronchial ultrasound (EBUS) can also be used for targeting.
Peripheral tumor
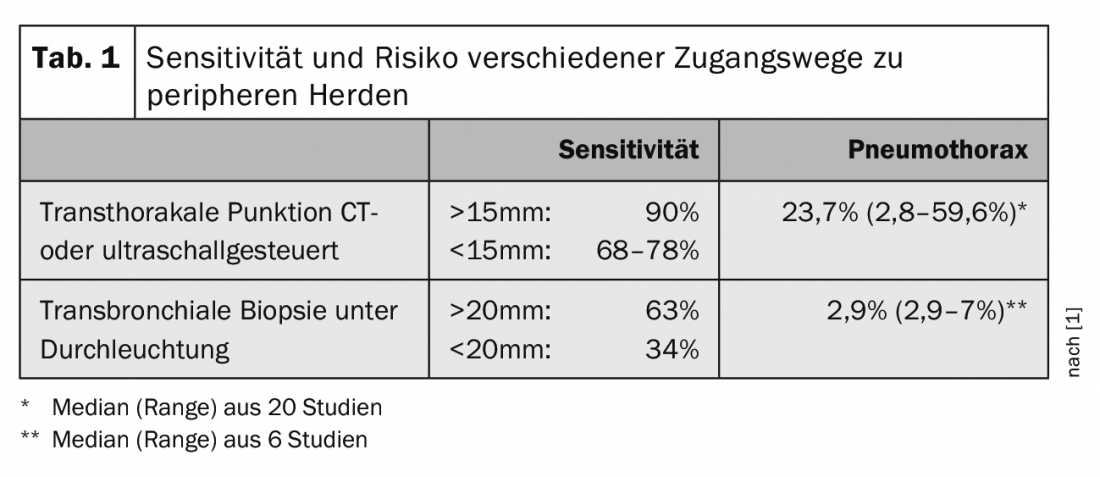
There are two possible access routes to peripheral tumor manifestations, which differ in terms of diagnostic yield and risk (Tab. 1) : Transthoracic puncture has a high sensitivity. For foci in contact with the chest wall, it can be ultrasound-guided in the hand of the pulmonologist. If there is air-containing lung tissue between the focus and the chest wall, CT-guided access by the radiologist is required. However, because of the necessary injury to the pleura, there is a relevant risk of pneumothorax. Bronchoscopically guided transbronchial biopsy has lower sensitivity, especially for smaller foci. However, because it is performed via naturalis, it is also associated with a significantly lower pneumothorax rate. Therefore, the bronchoscopic approach should be preferred whenever possible, especially since it allows the evaluation of other thoracic tumor manifestations in the same procedure. Individual selection of the optimal method requires a detailed study of the chest CT in the lung window, paying particular attention to the positional relationship of the focus to the bronchial tree and the presence of a feeding bronchus. A high resolution in the form of a low layer thickness (≤1 mm) is of great advantage.
Prior to the transbronchial biopsy, the examiner must mentally plan the access route as accurately as possible on the chest CT. A thin bronchoscope (<5 mm) should be chosen to allow biopsy instruments to be steered as far peripherally as possible. Manufacturers have now also developed ultrathin bronchoscopes (≤3 mm) with sufficient working channel for this indication. In addition, attention should be paid to indirect evidence of tumor localization in the area visible by bronchoscopy, such as traces of blood, compression phenomena, and secretion in ostia.
Probing beyond the area that can be viewed bronchoscopically requires the use of another procedure to navigate and target the biopsy instrument. X-ray fluoroscopy is classically used for this purpose. A co-movement of the focus in the fluoroscopic image at the moment of biopsy (“wiggle sign”) may indicate targeting. A combination of biopsy instruments (forceps, cytology brush, TBNA needle) may increase diagnostic yield. However, the sensitivity drops rapidly in proportion to the size of the peripheral focus (Table 1).
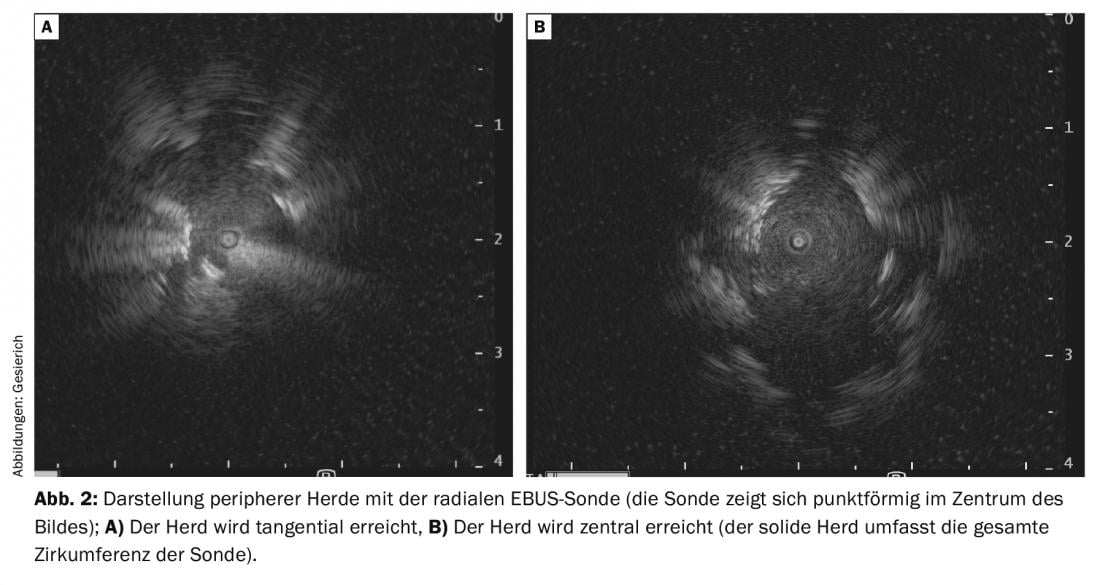
This is spurring the development of a variety of other navigation aids [2]. Due to its low cost and time-saving application, the radial ultrasound probe (rEBUS) has found the most widespread use. A rotating transducer at the tip of a flexible probe provides a 360° image of the bronchial environment. On the way to the focus, the typical sonographic picture of air-containing lung parenchyma is seen. If the focus is reached, it is delineated in the ultrasound image. A tangential position at the focus must be distinguished from a central position inside the focus (visualization of solid tissue in the entire circumference of the probe) (Fig. 2A and B) . The latter situation is associated with a higher diagnostic yield. The path thus found is then traced with the biopsy instrument, using the position of the rEBUS probe in the fluoroscopic image as a reference. To facilitate targeting, the probe can also be used to advance a guide catheter to the focus, through which biopsy instruments are then inserted after the probe is withdrawn. This procedure also allows the use of ultrathin cryoprobes for transbronchial biopsy. This allows large hemispherical biopsies to be obtained that also contain tangential portions of the bronchial environment, in contrast to a more orthograde forceps biopsy. Therefore, this method should be considered especially in case of only tangential visualization of the peripheral focus in the rEBUS.
A much more costly and time-consuming method is electromagnetic navigation (EMN). The patient lies in an electromagnetic field generated by a board integrated into the examination couch. Navigation is performed with a probe at the tip of which induction currents are induced in coils that vary according to their position in the electromagnetic field and the extent of which allows localization. The patient’s chest CT must first be read into the navigation system like a “road map”, and the position of the probe tip is then displayed three-dimensionally in the CT image. Also with this method, a guiding catheter is carried to the peripheral focus, through which biopsy can then be performed. Finally, there is the option of virtual bronchoscopy (VB): software reconstructs a virtual bronchial tree from the data set of a high-resolution chest CT, through which a path to the peripheral focus can be semi-automatically planned in advance. During real bronchoscopy with an ultrathin bronchoscope, there is a continuous, software-assisted comparison between the virtual and endoscopic images. At the bronchial division sites, the software displays the subsegment to be selected.
With the described navigation aids, a sensitivity of up to 70% can be achieved even in smaller peripheral foci [3]. The technical accessibility of a bronchus remains a limitation. Therefore, there are experimental attempts to leave the bronchial tree and proceed transparenchymal to the peripheral focus (“bronchoscopic transparenchymal nodule access”, BTPNA). This involves using virtual bronchoscopy to pre-plan a “point of entry” (POE) into the airway wall and a pathway through the parenchyma to the focus that is as free of vessels as possible. Bronchoscopy is then used to puncture at the POE and create a tunnel to the peripheral focus via a trocar.
At present, the use of robot-assisted bronchoscopy systems may be regarded as highly experimental and probably very cost-intensive. The “Ion Platform” (Intuitive Surgical, Sunnyvale, CA, USA) consists of a flexible catheter with a working channel of 2 mm, the tip of which can be angled in all planes in finely controlled movements. The entire length of the catheter is traversed by a sensor fiber that provides feedback on shape and position. The examiner can remotely control the system via a console using a track ball and scroll wheel, which is translated by the robot into precise and cleaned probe tip movements. Further targeting is similar to virtual bronchoscopy, with the sensor fiber providing additional information for navigation and positional stability during biopsy [4].
In connection with the expected introduction of lung cancer screening, a large number of peripheral foci requiring clarification can be expected, although the majority of these findings will be benign. In this context, too, further development of the bronchoscopic instrumentation for peripheral probing is to be welcomed in order to enable the most minimally invasive clarification of these findings.
In some tumor locations difficult to reach for transbronchial biopsy, access can be found using transbronchial needle aspiration via an ultrasound puncture bronchoscope (EBUS-TBNA). This applies to paramediastinal tumors of both upper lobes adjacent to the trachea and esophagus, as well as to peribronchial and hilar tumors.
Mediastinal lymph node status
Mediastinal lymph node status is the most important prognostic factor in nonremote metastatic patients and a crucial parameter for determining operability in lung cancer. Therefore, the most accurate pretherapeutic detection of mediastinal lymph node involvement is of great importance for treatment planning.
Imaging methods including PET-CT have insufficient sensitivity and specificity for this purpose (Tab. 2). Invasive staging is therefore required.

Due to the large range and the associated high sensitivity and specificity as well as the lower risk, endosonography and ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) are now recommended in the guidelines as the minimally invasive method of first choice for mediastinal staging, whereas invasive surgical access routes (mediastinoscopy, video-assisted thoracoscopy) have been given reserve status for unclear situations.
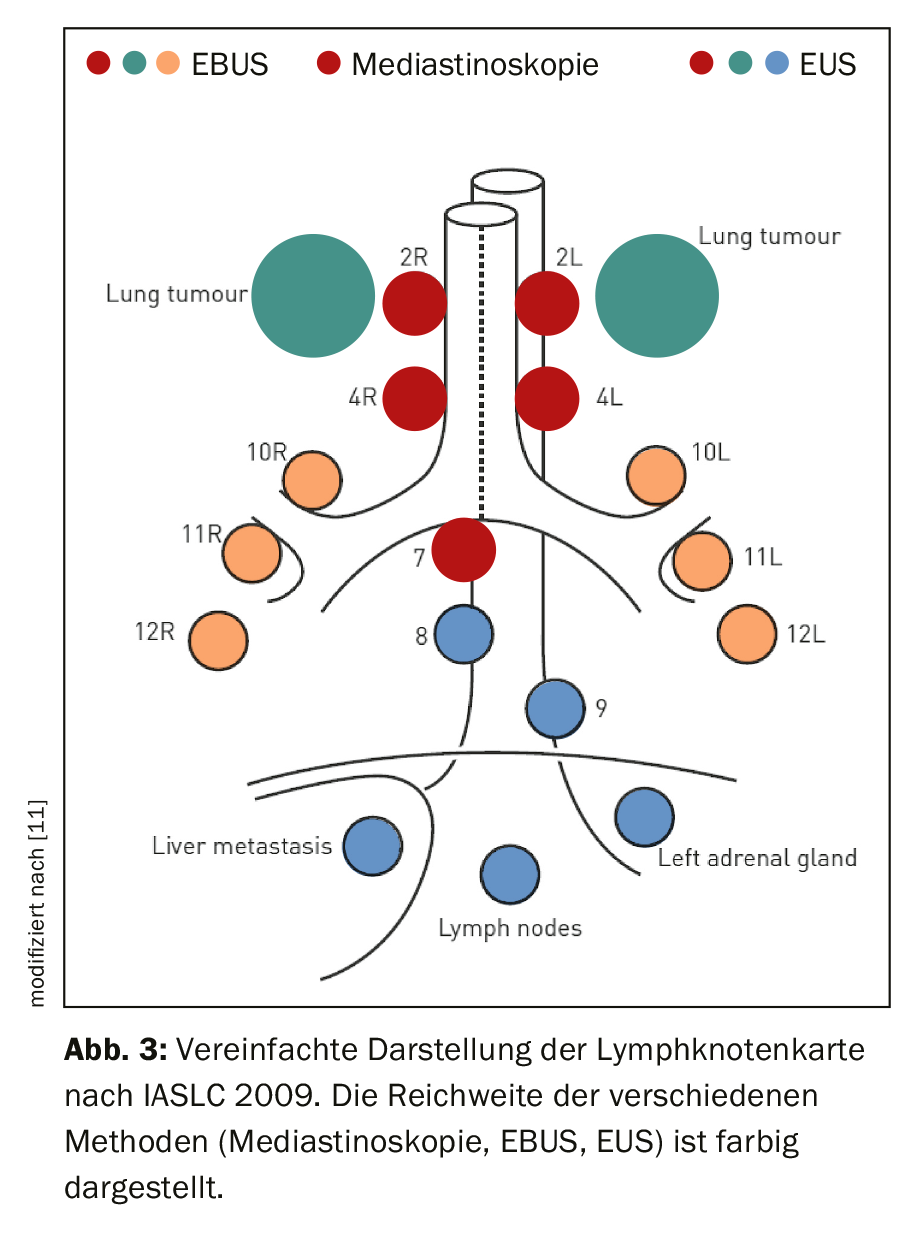
The examiner requires a detailed knowledge of the anatomy of the thoracic lymph nodes. For this purpose, the lymph node map of the International Association for the Study of Lung Cancer (IASLC) is applied in its 2009 proposed version, details of which can be found in the corresponding publication [6,7]. Individual lymph node stations are defined in terms of their topographic relationship to the tracheobronchial tree and thoracic vessels. Station 1 corresponds to the supracalvicular lymph nodes and is therefore assigned to the N3 level. Stations 2-9 are located from cranial to caudal in the mediastinum. They are assigned to the N2 level if the tumor is ipsilateral, and to the N3 level if the tumor is contralateral. Station 10 is located at the hilus, and stations 11-14 are located lobar, segmental, and subsegmental in the lungs. These belong to the N1 level in the case of ipsilateral tumor and to the N3 level in the case of contralateral tumor. Figure 3 shows the selection of endosonographically accessible lymph node stations. While mediastinoscopy covers only the upper and lower paratracheal lymph nodes (stations 2 and 4) and the infracarinal lymph nodes (station 7), endobronchial ultrasound (EBUS) additionally reaches the hilar (station 10), interlobar (station 11), and segmental (station 12) lymph nodes. Transesophageal access (endoscopic ultrasound; EUS) can also be used to puncture the lower mediastinal lymph nodes (stations 8 and 9).
Endosonographic staging should be complete and systematic. The guidelines of the European Respiratory Society (ERS) recommend the combination of EBUS and EUS, if available, as this is the only way to achieve the full range of endosonography [8]. EUS examination is usually performed with an ultrasound gastroscope in the hand of the gastroenterologist. However, the ultrasound bronchoscope (EUS-B) can also be inserted through the esophagus. This requires appropriate training of the examiner, but allows complete endosonographic staging by the pulmonologist in one procedure with a correspondingly lower patient burden and cost. The guidelines recommend at least one needle aspiration from the lower paratracheal (stations 4R and 4L) and infracarinal (station 7) lymph nodes. In addition, all stations that are radiologically and/or endosonographically conspicuous should be sampled. For endosonography, a size threshold of 5mm has often been applied in studies for this purpose. Because EBUS-TBNA is a cytological method, sampling must begin at the N3 level and progress through the N2 level to the N1 level. Deviation from this procedure could result in upstaging due to carryover of malignant cells from lower-ranking tumor-infested lymph node stations via the puncture needle into sample vessels assigned to higher-ranking stations.
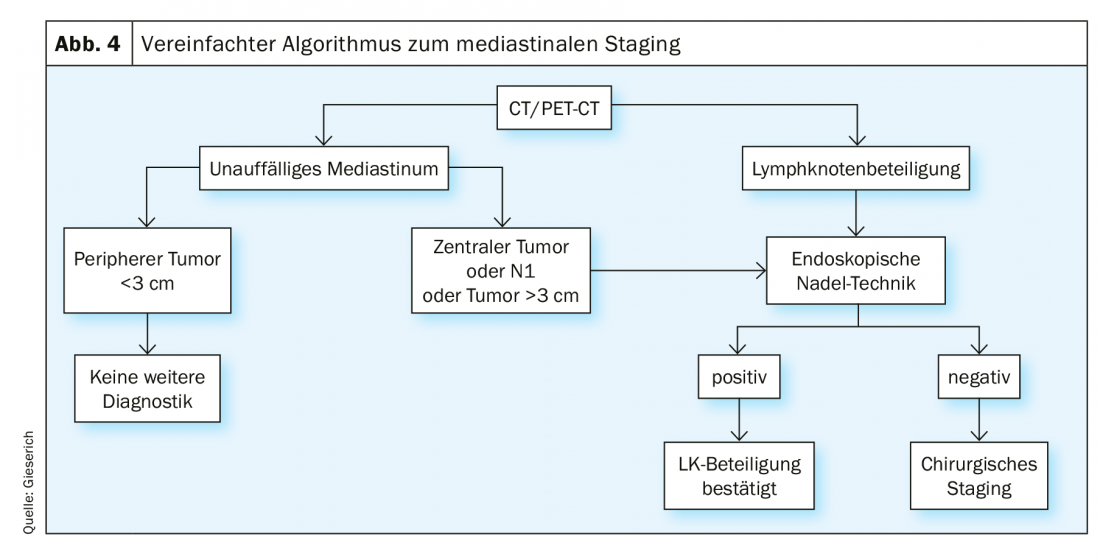
Endosonographic staging [8] is indicated in all cases with radiologically abnormal mediastinum, defined by mediastinal lymph nodes >1 cm on CT and/or with metabolic activity on PET. If the mediastinum is radiologically unremarkable, there is an indication in the following situations: central location of the primary tumor, radiological suspicion of lymph node metastases at the hilus (N1 situation), and a peripheral tumor >3 cm. If the endoscopic needle technique provides a positive result, the lymph node involvement may be considered confirmed. If no tumor cells are found, a false-negative result cannot be excluded. The incidence of this is reported in the literature to be up to 24% [1]. If relevant for the therapeutic decision, invasive surgical staging must follow in this situation (Fig. 4).
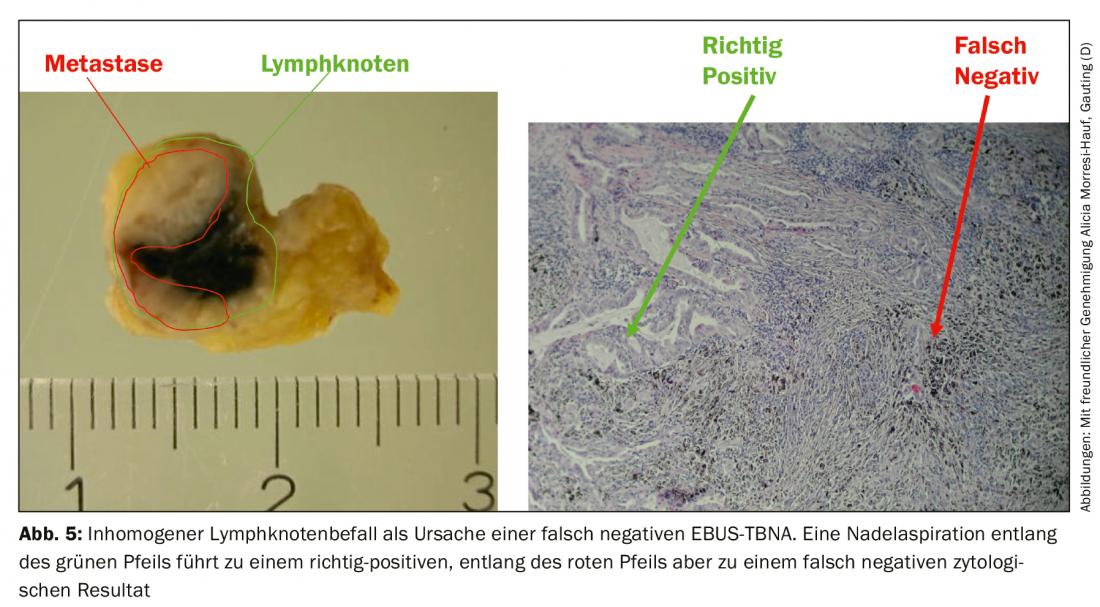
The false-negative rate results from inhomogeneous tumor involvement of the lymph nodes, whereby in unfavorable cases the needle only captures healthy lymph node parenchyma (Fig. 5) . In addition to comparison with the radiological findings, endosonography also provides criteria for further classification of negative needle aspirates. First, characteristics of the conventional EBUS image associated with malignancy have been described: Whereas benign lymph nodes tend to have an oval shape with a fuzzy border and typical vascular hilus, malignant nodes tend to assume a roundish shape, the border becomes more sharply defined, the central hilar vessel may be absent, and the echo texture may become inhomogeneous due to coagulation necrosis. On the other hand, elastography can be used as a still experimental sonographic method. The method represents the deformability (elasticity) of the examined tissue. In the thoracic application, the deformation of the lymph nodes is exploited by the regular pulsation of the heart and the great vessels. Similar to the flow velocity in duplex ultrasonography, the deformability is transferred to a color scale and superimposed on the ultrasound B-scan. The color representation changes from high to low elasticity from red to yellow and green to blue. The prediction of malignancy is based on the assumption that the lymph node tissue loses its elasticity due to carcinoma infiltration. Malignant lymph nodes therefore appear predominantly blue (Fig. 6A), benign ones predominantly non-blue. The investigator should indicate these findings – if available – in the examination report and bring them into the discussion of the tumor conference.

In situations with lack of bronchoscopic accessibility to the peripheral primary tumor, mediastinal and hilar lymph node metastases may be the only accessible tumor manifestations. Therefore, it is important to emphasize that all necessary histopathological examinations, including molecular analyses, can be performed on needle aspirates obtained by endosonography. This requires high-quality specimen processing in the pathology laboratory, which includes not only smear preparations but also the preparation of a cell block by centrifugation, enrichment of the cytological material and kerosene embedding.
Metastases
Distant metastases usually stand in the way of curative therapy. Therefore, in radiologically unclear situations, the need for histological clarification arises here as well. While brain and bone metastases usually require reliance on imaging findings, other metastatic sites are more amenable to specimen collection.
If a pleural effusion occurs in the context of a lung carcinoma, the first step is a cytological examination of the pleural punctate. Since the sensitivity is only about 50%, the next step in case of negative cytology and therapeutic relevance must be video-assisted thoracoscopy to exclude pleural carcinomatosis with certainty.
In the case of space-occupying lesions on the adrenal gland, the sensitivity and specificity of imaging diagnostics are not sufficient for a definitive diagnosis, so that histological confirmation may be desirable, especially in the case of isolated metastasis. The classic access route is CT-guided transcutaneous puncture. Endosonographic access via the upper gastrointestinal tract allows transgastric puncture of the left adrenal gland in most cases; in some cases, the right adrenal gland can be reached transduodenally. This is usually done in the hands of the gastroenterologist using an ultrasound gastroscope. Feasible, but still experimental, is ultrasound bronchoscope access to the left adrenal gland (Fig. 6B and C), again making the procedure accessible to the pulmonologist and allowing it to be performed in the same session with bronchoscopy [9]. There are also single case reports in the literature of securing liver metastases and metastases in the epigastric lymph nodes by the same route.
Procedural aspects
The described requirements for modern bronchoscopy in lung cancer give an idea of the complexity of the procedure. In principle, all the methods listed can be used in purely flexible technology. Difficult peripheral probing and comprehensive endosonographic staging, however, are associated with corresponding time requirements and require quiet examination conditions. A modern bronchoscopy unit must therefore have the capability to perform the examination under general anesthesia. The technique of rigid bronchoscopy is optimal for this purpose. If this is not available, the laryngeal mask can be selected as the ventilation access. This allows free movement of the flexible bronchoscopic instruments – especially the ultrasound puncture bronchoscope – in the trachea, so that the paratracheal lymph node stations also remain accessible.
In addition, high demands are placed on the qualifications of the investigator. As experience increases, the diagnostic yield increases and the complication rate decreases. Simulators exist for endosonography, as well as rubber or animal organ models that allow the trainee to master the first part of the learning curve. The next step is the gradual assumption of the examination on the patient under supervision. Because the learning curve may vary in steepness from individual to individual and the specification of minimum examination numbers is arbitrary, the literature [10] suggests standardized, validated tests that can be used to assess the bronchologist’s competence prior to independent examination.
Take-Home Messages
- Bronchoscopy is the method of choice for histological confirmation of lung carcinoma. It allows a comprehensive evaluation of the thoracic situation including central and peripheral tumor manifestations and mediastinal lymph node status.
- The cryoprobe is gaining increasing importance both as an instrument for central and transbronchial biopsy and for recanalization in exophytic airway stenosis.
- Navigational aids for probing peripheral foci are in rapid development, from the simple and inexpensive to use radial ultrasound probe, to more complex techniques such as electromagnetic navigation and virtual bronchoscopy, to experimental approaches such as transparent chymal access and robotic-assisted bronchoscopy.
- According to the 2009 IASLC map, thoracic lymph node stations are divided into 14 stations, the anatomy of which must be mastered by the examiner.
- In mediastinal staging guidelines, endosonographically guided needle techniques are now considered first-line methods because of their high sensitivity and low complication rate.
- Full coverage is achieved by combined access via the respiratory tract (EBUS) and esophagus (EUS). Both can be done in one procedure using the ultrasound bronchoscope.
Literature:
- Rivera MP, Mehta AC: American College of Chest P: Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132: 131S-148S.
- Ishiwata T, Gregor A, Inage T, Yasufuku K: Advances in interventional diagnostic bronchoscopy for peripheral pulmonary lesions. Expert Rev Respir Med 2019; 13: 885-897.
- Wang Memoli JS, Nietert PJ, Silvestri GA: Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012; 142: 385-393.
- Fielding DIK, Bashirzadeh F, Son JH, et al: First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019; 98: 142-150.
- Silvestri GA, Gonzalez AV, Jantz MA, et al: Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e211S-250S.
- Rusch VW, Asamura H, Watanabe H, et al: The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009; 4: 568-577.
- Tournoy KG, Annema JT, Krasnik M, et al:. Endoscopic and endobronchial ultrasonography according to the proposed lymph node map definition in the seventh edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2009; 4: 1576-1584.
- Vilmann P, Clementsen PF, Colella S, et al: Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015; 47: c1.
- Crombag LM, Annema JT: Left Adrenal Gland Analysis in Lung Cancer Patients Using the Endobronchial Ultrasound Scope: A Feasibility Trial. Respiration 2016; 91: 235-240.
- Konge L, Vilmann P, Clementsen P, et al: Reliable and valid assessment of competence in endoscopic ultrasonography and fine-needle aspiration for mediastinal staging of non-small cell lung cancer. Endoscopy 2012; 44: 928-933.
- DOI http://dx.doi.org/10.1055/s-0034-1392040 Published online: 0.0. Endoscopy 2015; 47: 545-559 © Georg Thieme Verlag KG Stuttgart – New York ISSN 0013-726X
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 1(3): 6-11.