Atrial fibrillation is the most common cardiac arrhythmia in the Western world. Interventional treatment shows promising results. A key role is played by pulmonary vein isolation (PVI). Furthermore, there are news like the so-called “high-density mapping” or the localization and visualization of rotors.
AF is the most common cardiac arrhythmia in the Western world with a prevalence of 1.5-2%, currently affecting an estimated 34 million people worldwide [1]. The Framingham cohort was studied in relation to lifetime risk for AF and found that 25% of men and women >40 years will develop AF in their lifetime [2]. As society ages, the prevalence of AF is expected to double in the next 50 years. AF is associated with increased risk of stroke, heart failure, hospitalizations, but also increased mortality, and therefore represents a particularly important medical, economic, and social challenge. In addition to pharmacological therapy, which is often ineffective and associated with adverse side effects, interventional catheter treatment of AF has become the standard therapy for rhythm control in current guidelines because of its efficacy [3]. Whether interventional therapy for AF leads to reduced mortality in addition to improved morbidity is the subject of ongoing multicenter randomized trials (CABANA, EAST) [3].
Atrial fibrillation ablation
After venous inguinal punctures and one or two transseptal punctures through the atrial septum from the right to the left atrium, a three-dimensional electroanatomic reconstruction of the left atrium is now commonly obtained for the most commonly used radiofrequency ablation (Fig. 1), which can significantly reduce fluoroscopy time. In many centers, this reconstruction is supported with prior computed tomography or magnetic resonance imaging. Special attention must be paid to reconstruction of the pulmonary veins (PV), left atrial auricle (LAA), and mitral annulus (MA). Selective angiography of the PV may be helpful in this regard. Three-dimensional mapping systems can be used to generate electrical voltage (voltage) and activation maps, providing valuable information about the presence of a pathological substrate in the left atrium and about mechanisms and origins of atrial arrhythmias. Diagnostic catheters for derivation of electrical signals and stimulation are usually placed in the coronary sinus and PV. Ablation of AF is performed under therapeutic heparinization. Complications of AF ablation are rare overall; the most common complications include groin complications in approximately 2-4% (hemorrhage, arteriovenous fistula/aneurysm), followed by pericardial effusions/pericardial tamponades (approximately 1-2%) and transient ischemic attacks/strokes (<1%). The most feared complication is an iatrogenic fistula between the esophagus and the left atrium (atrioesophageal fistula), which usually manifests a few weeks after ablation, is very rare overall (<0.5%), but is often lethal [3].
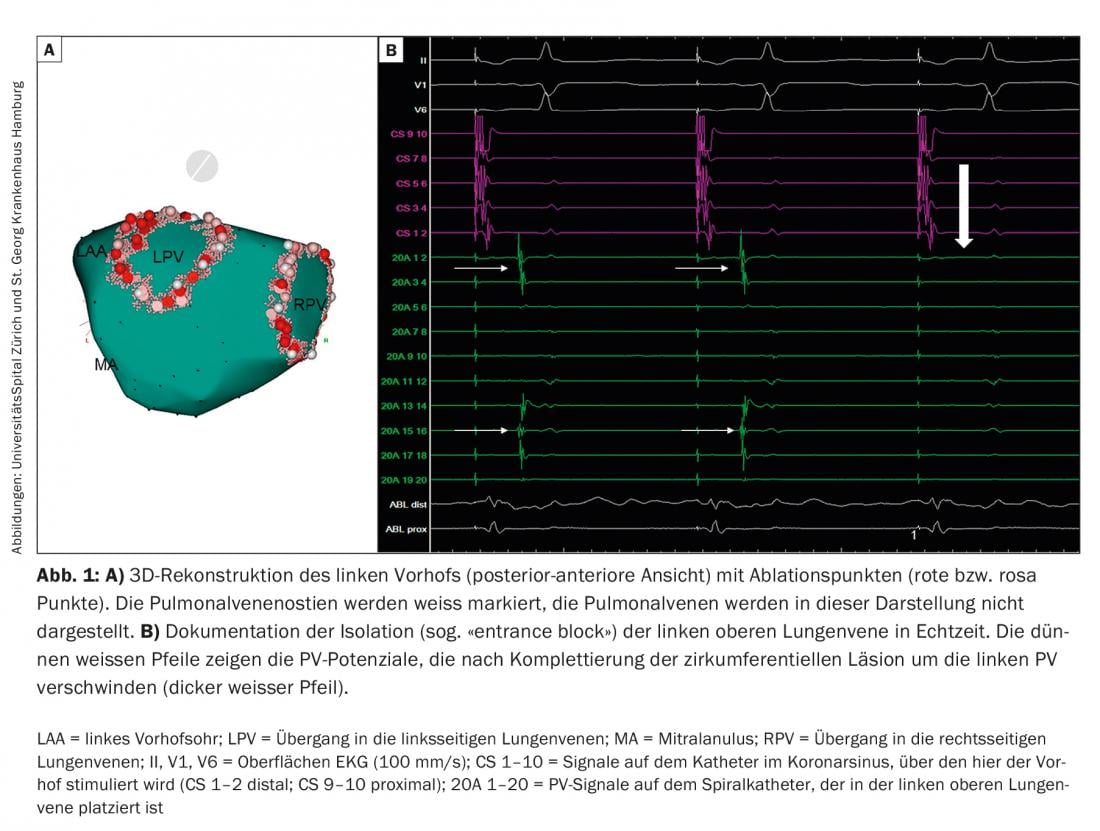
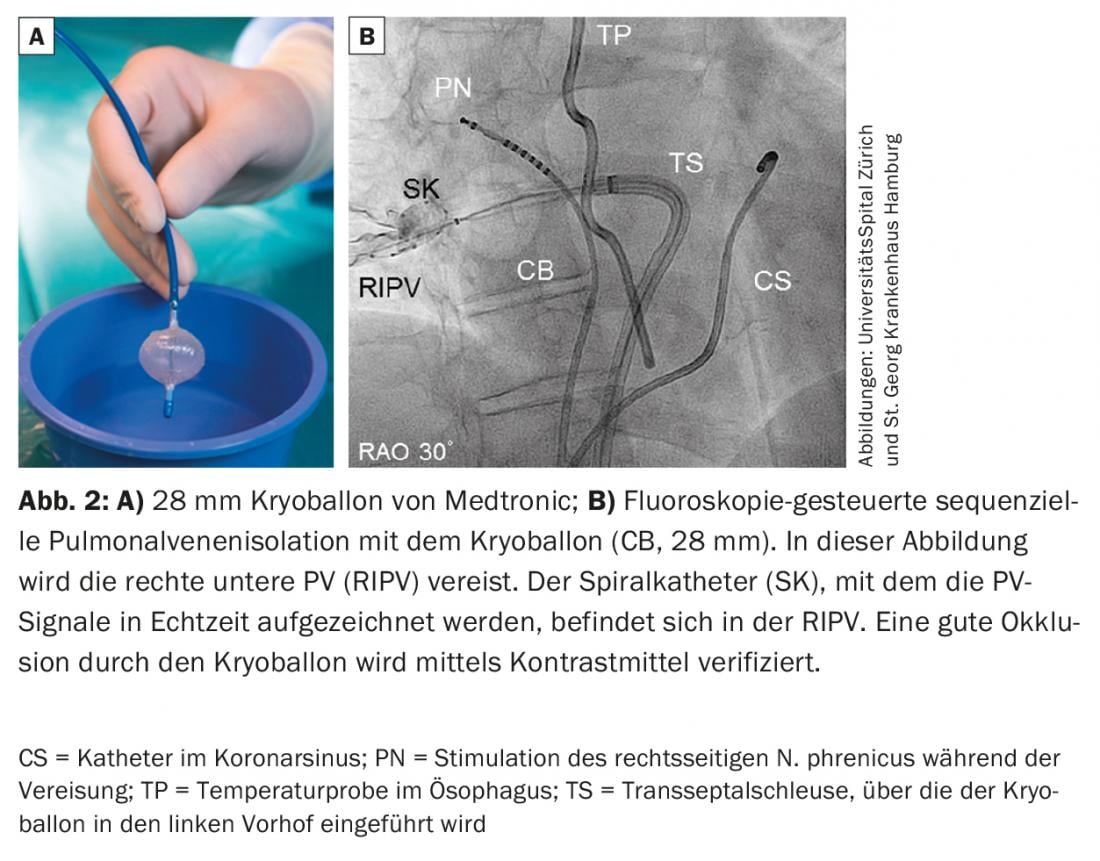
Paroxysmal atrial fibrillation (PAF): ectopic foci are responsible for triggering AF [4]. Because the PVs are connected to the left atrium via cardiac muscle fibers, electrical actions can be transmitted to the atria via these fibers and trigger AF here. This realization has fundamentally changed our understanding and treatment strategies of AF. Whereas cardiac surgical therapy for AF in the 20th century was aimed at eliminating or reducing the substrate of AF-the atrial tissue that sustains AF-today’s therapeutic strategies, particularly in PAF, are aimed at eliminating the triggers. Because these triggers most commonly arise in the PV, pulmonary vein isolation (PVI) has emerged as the current main invasive therapeutic strategy for AF. Today, the electrical “wide-area” circumferential PVI using radiofrequency current (Fig. 1), alternatively the electrical isolation of the PV with the cold balloon (cryoballoon; Fig. 2) or laser balloon, represent the cornerstone of interventional therapy for AF. Especially in patients with PAF, PVI can lead to stable sinus rhythm for several years in the majority of patients. This is true for younger adults <45 years [5], but also for patients >80 years. However, in some patients, it requires multiple procedures. Our experience has shown that long-term freedom from AF/atrial tachycardia (AT) over a median follow-up period of five years after an intervention was present in 47% of patients. However, after several interventions (median 1, range 1-3), this number was increased to 80% [6]. After a median follow-up period of ten years, freedom from AF/AT after multiple ablations is approximately 63%. Importantly, here the main reason for recurrent AF is electrical reconnection of the PV to the left atrium, even when only one PV has renewed electrical conduction. This electrical reconnection of the PV is found in up to 92% of patients with AF who are assigned to repeat PVI. In these cases, re-isolation of PV can lead to clinical control of AF in 81% of patients. However, additional non-PV AF triggers were identified in 14% of these patients.
Currently, there is little data to suggest further empirical ablation targets in addition to PV in patients with PAF. The same applies to re-procedures for the treatment of PAF in which the PVs were re-isolated. Occasionally, however, the additional elimination of triggers outside the PV, such as from the superior vena cava, posterior wall of the left atrium, or crista terminalis, may lead to an improved outcome of PVI in PAF.
Persistent AF: The longer AF persists, the more pronounced the structural changes (“remodeling”) of the atria. This leads to a vicious cycle: AF causes AF (“atrial fibrillation begets atrial fibrillation”) [7]. Chaotic electrical excitation and uncoordinated contractions of the atria result in changes in the electrical properties of the atrial myocardium, reduced contractility, dilatation, and atrial fibrosis. This favors the maintenance of the AF. While focal activity from the PV is crucial as a trigger in PAF, it recedes into the background in persistent AF. Therefore, the published success rates of PVI for persistent AF are generally lower than for PAF, but remain significantly better than drug-only therapy. To improve the outcomes of catheter ablation of persistent AF, new therapeutic strategies have been developed. These are based on multiple theories of formation mechanisms of (persistent) AF. One of the oldest theories is the so-called “multiple wavelet” hypothesis. This states that AF is generated and maintained by multiple independent and chaotic excitation waves moving in both atria. Because a minimum number of concurrent waves and a certain critical mass of excitable atrial tissue must be present to maintain AF, various surgical techniques (eg, Cox-Maze operation) have been developed in which multiple transmural incisions are made in both atria, thereby preventing intraatrial reentry. This technique was adapted decades later for endocardial catheter ablation of persistent AF.
Another hypothesis for the development of persistent AF is based on so-called CFAE (complex fractionated atrial electrograms). CFAE represent specific regions of the atria where there is a delay in electrical excitation (“slow conduction”), which may result in local microreentry. Therefore, these regions also lend themselves to catheter ablation.
In addition to eliminating the triggers in the PV, the stepwise ablation approach additionally aims at modifying the substrate in the LA. In this procedure, radiofrequency energy is first used to isolate the PV, followed by CFAE ablation. Thereafter, linear lesions are applied to the roof of the LA and to the MA until the termination endpoint of AF or transition to AT is reached and the latter is ablated and converted to sinus rhythm. Importantly, gaps in linear lesions can lead to delayed conduction of excitation in these areas, resulting in reentry and iatrogenic ATs. Therefore, the detection of an electrical line block along the applied line in both directions (so-called bidirectional block) is very important. In two recently published studies using this approach, freedom from AF/AT after one procedure and a mean of 2.1 procedures was 17/20% and 56/63%, respectively, after a follow-up of five years. Significant predictors of recurrent AF/AT were lack of conversion of AF to sinus rhythm during ablation, a left atrial diameter ≥50 mm, long-standing AF >18 months, and structural heart disease [8].
Our strategy for persistent AF is to first perform a “wide-area” circumferential PVI. Additional substrate modification is performed only if the patient cannot be converted to sinus rhythm (even by electrocardioversion). After a follow-up period of five years, the success rate with this strategy in patients with long-standing AF after initial intervention was 20%, and after a median of two interventions it was 45%. Importantly, patients with persistent AF <2 years in particular had good long-term outcomes.
In the recently published largest randomized multicenter trial of ablation of persistent AF (STAR AF 2), three ablation strategies were compared. 589 patients selected for first-time radiofrequency ablation because of persistent (<3 years) drug-refractory AF were randomized to the following treatment arms: PVI alone (group 1) vs. PVI + CFAE ablation (group 2) vs PVI + CFAE ablation + linear lesions (group 3). After an observation period of 18 months, no statistically significant differences were shown between the treatment arms.
What do the guidelines say?
The current 2016 European guidelines on AF list catheter ablation of symptomatic AF in experienced centers as a clinically established method that should usually be performed after an unsuccessful treatment attempt with antiarrhythmic drugs (class IA recommendation for PAF and IIa B for persistent AF). The primary goal here is PVI, which can be achieved using radiofrequency current or the cryoballoon (IIa B). In symptomatic patients, primarily those with PAF without significant structural heart disease or with persistent AF with suspected tachycardiomyopathy, catheter ablation is also recommended as an alternative to drug antiarrhythmic therapy because of its good success rates and low complications (IIa B). In structural heart disease, it is noted that more extensive ablation of the LA may be required. Ablation of the right atrial isthmus should be performed as part of AF ablation if isthmus-dependent atrial flutter has been documented before or during the procedure (IB). Catheter ablation should be followed by heparin administration and overlapping oral anticoagulants for at least two months (IIa B). Periprocedurally, the new oral anticoagulants (NOAC) may also be used. Thereafter, the risk factors for stroke should be the basis for further decision on anticoagulation (IIa C). Sustained continuation of anticoagulation should be considered in patients with one major or two clinically relevant nonmajor risk factors for stroke (eg. B. CHA2DS2-VASc score ≥2) (IIa B).
Decisions regarding cardiac surgery to treat more complex forms of AF after multiple unsuccessful interventional treatments should be made on the recommendation of an AF heart team. At a minimum, an AF heart team should include an interventional electrophysiologist, a cardiac surgeon with experience in surgical AF ablation, and a cardiac anesthesiologist [3].
Modern developments in interventional therapy
contact pressure (“contact force”): Reconducting the PV after successful isolation (reconnection) is one of the key challenges of PVI. This is due in no small part to insufficient lesions during the index procedure. It is well known that the contact force (“CF”) from the catheter tip to the tissue to be sclerosed is very important to be able to set transmural lesions. Initial studies investigating these novel catheters with the ability to measure CF and a cooled catheter tip demonstrated their safety for AF ablation. It was also shown that results were dependent on the level of CF. A mean CF <10 g was significantly more frequently associated with recurrent arrhythmias, whereas above-average results were obtained with a CF >20 g (however, the risk of complications increased at >40 g). During a diagnostic second procedure after initial ablation with CF catheters, incomplete tissue lesions correlated inversely with CF and the product of CF and ablation duration (so-called force-time integral, FTI). The EFFICAS I study led to an adjustment of recommendations indicating an optimal CF of 20 g and a minimum of 400 gs (FTI) per ablation lesion during ablation.
Cryoballoon: The cryoballoon (Fig. 2) was introduced in 2006 and has proven to be a safe and effective system for PVI. Electrical PVI is achieved by positioning the balloon catheter at the ostium of the respective PV and cooling it to -55°C with liquid nitrogen so that the tissue at the junction of the left atrium and PV freezes (usually for 180-240 s per PV). The balloon catheter is inserted into the LA through a steerable long sheath. A multipolar circular mapping catheter is inserted via the lumen of the balloon catheter to derive signals from the PV. With the introduction of the second generation, long-term outcomes were significantly improved over the first generation to freedom from AF/AT of >80% at one year and >70% at two years after an index procedure. A characteristic complication of cryoballoon is right phrenic nerve paresis. Phrenic nerve palsy is usually reversible within one year, but persists in 2.5% of patients. The rate of phrenic nerve paresis could be reduced if icing was performed with less contact pressure, the balloon catheter was positioned more antrally, and the phrenic nerve was stimulated during icing of the right PV to detect motility disturbances and a reduction in electrical activity of the right diaphragm in a timely manner. Several studies (including the recently published largest multicenter prospective randomized trial of AF ablation [9]) have demonstrated that the cryoballoon is equivalent to PVI in terms of efficacy and safety compared with the older technology of radiofrequency ablation.
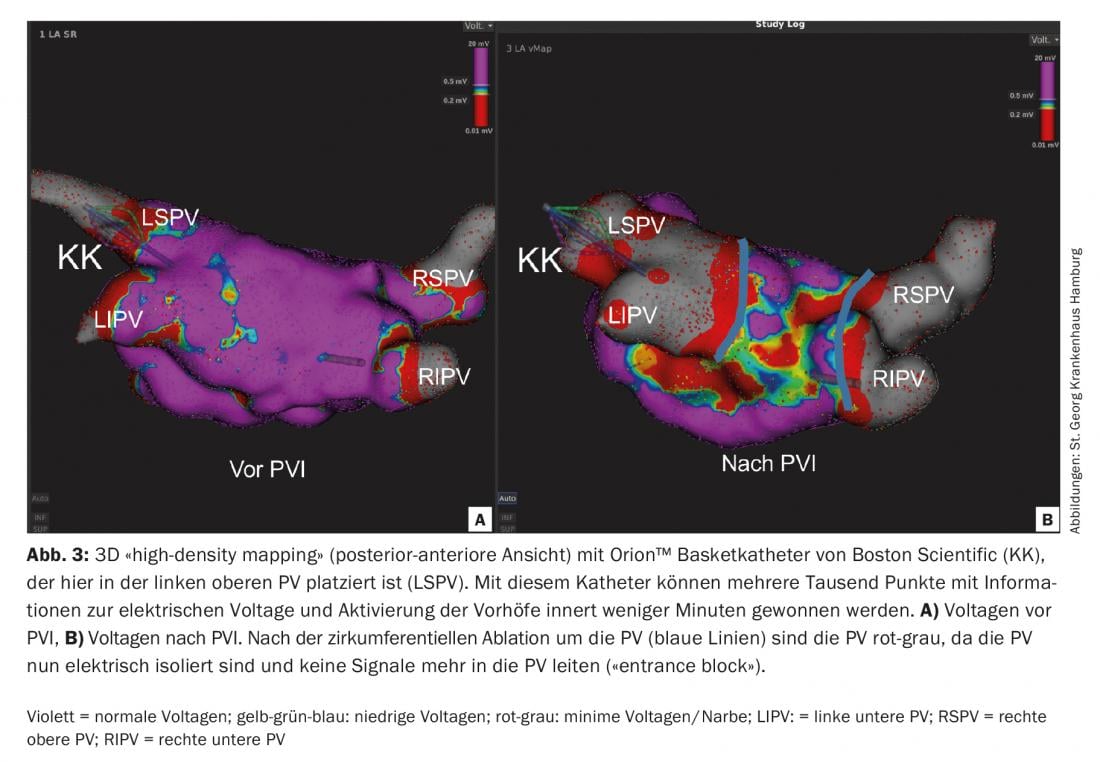
“High-density mapping: With the so-called “high-density mapping” it has recently become possible, within a few minutes, to use multipolar diagnostic catheters (e.g. Orion™ from Boston Scientific, or Pentaray® by Biosense Webster) to create a 3D electroanatomical map of the atria with several thousand points without X-rays (Fig.3). These 3D maps provide very accurate information about voltage potentials as well as electrical activation of the myocardium, which can be particularly helpful in ablation of atrial reentry tachycardia [10].
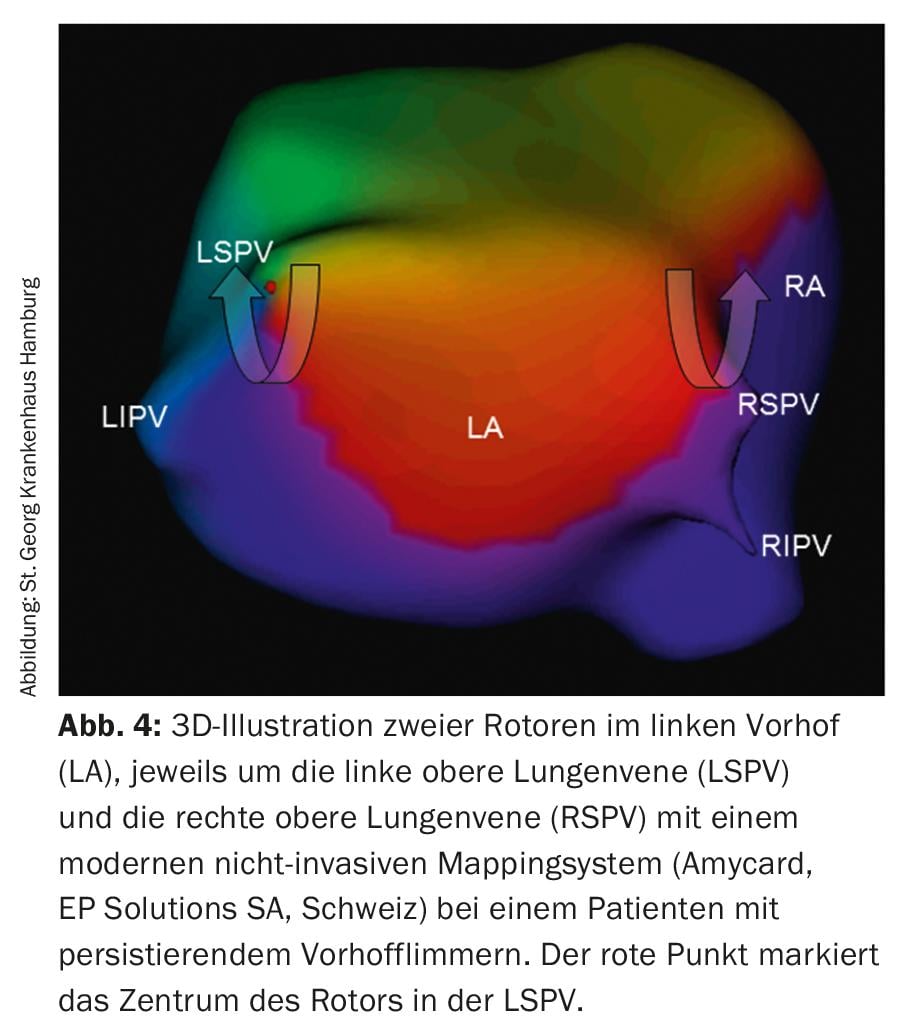
Ablation of rotors: A newer concept on the causes of persistent AF is represented by so-called rotors (Fig. 4) in the atria, which initiate and maintain AF – like a hurricane – and may therefore also be the target of catheter ablation. Currently, various systems exist to enable the localization and visualization of rotors. However, long-term results of this new technology have to be awaited.
Risk Factor Control: A completely different approach to improving long-term prognosis after AF ablation was taken by the Aggressive Risk factor REduction STudy for Atrial Fibrillation (ARREST-AF). Here, 149 patients with AF and a body mass index of at least 27 kg/m2 and at least one additional cardiac risk factor were ablated. Sixty-one chose to participate in rigorous management of risk factors under the supervision of physicians at a specialized clinic; the remaining 88 subjects were taught about rigorous management of risk factors only. During the follow-up period of 3.5 years, these measures led to a significant improvement of the risk profile in the group with risk factor management compared with the control group on the one hand, and on the other hand, the ablation success also lasted longer: 33 vs. 10% arrhythmia freedom after single ablation, 87 vs. 18% after multiple ablation [11].
Take-Home Messages
- Atrial fibrillation (AF) is the most common cardiac arrhythmia in the Western world, with a prevalence of 1.5-2%, and thus represents a
- important clinical and socioeconomic challenge.
- Interventional treatment of AF shows promising results, with pulmonary vein isolation (PVI) playing a key role.
- One challenge of catheter treatment of AF is the creation of transmural permanent lesions. New procedures such as the cold balloon (cryoablation) or contact sensor catheters should improve this.
- Especially in paroxysmal (PAF) and short persistent (<3 months) AF, the chances of success of PVI are good, but also longer persistent AF can be successfully treated with catheter ablation.
- Whether interventional therapy leads to reduced mortality in addition to improved morbidity is the subject of ongoing studies.
Conflicts of interest: Ardan Saguner: Lecture honorarium from Boston Scientific; Educational grants from Biosense Webster, Biotronik, Boston Scientific, and St. Jude Medical.
Literature:
- Chugh SS, et al: Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014; 129(8): 837-847.
- Lloyd-Jones DM, et al: Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110(9): 1042-1046.
- Kirchhof P, et al: 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37(38): 2893-2962.
- Haissaguerre M, et al: Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10): 659-666.
- Saguner AM, et al: Catheter ablation of atrial fibrillation in very young adults: a 5-year follow-up study. Europace 2016; euw378.
- Ouyang F, et al: Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010; 122(23): 2368-2377.
- Wijffels MC, et al: Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7): 1954-1968.
- Scherr D, et al: Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015; 8(1): 18-24.
- Kuck KH, et al: Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23): 2235-2245.
- Saguner AM, et al: First clinical experience using a novel high-resolution electroanatomical mapping system for left atrial ablation procedures. Clin Res Cardiol 2016; 105(12): 992-1002.
- Pathak RK, et al: Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation. Journal of the American College of Cardiology 2014; 64(21): 2222-2231.
CARDIOVASC 2017; 16(4): 24-29