With the increasing number of system therapeutics, practical advice on choosing the most suitable treatment option is becoming more important. The new edition of the S3 guideline on atopic dermatitis published this year contains numerous evidence-based indications for the use of biologics and Janus kinase inhibitors. Compared to the previous version, tralokinumab, as well as abrocitinib, baricitinib and upadacitinib have been included in the guideline alongside dupilumab and provided with corresponding information on the implementation of therapy.
When used appropriately, biologics and JAK inhibitors have great potential in the systemic treatment of patients with moderate to severe forms of atopic dermatitis (AD). What all these modern systemic active substances have in common is that they are particularly suitable for certain subgroups of patients and should be used as part of an individualized treatment plan. In order to achieve the best possible therapeutic results, it is advisable to weigh up the risk-benefit profiles both at the level of the substance classes and for each of the active substances individually [1]. The monoclonal antibodies currently approved for AD (dupilumab, tralokinumab) (Table 1) are administered subcutaneously and, unlike JAK inhibitors, do not require laboratory or instrumental tests either before the start of treatment or during treatment [1]. Three JAK inhibitors (abrocitinib, baricitinib, upadacitinib) are also currently approved for long-term therapy (Table 2) . These are available in oral dosage form, although screening is required before starting treatment and monitoring during therapy [1].
Determine AD severity based on objective and subjective parameters
Validated skin scores include the SCORAD (Scoring Atopic Dermatitis Index) and the EASI (Eczema Area and Severity Index) [2]. The maximum score, i.e. maximum severity of AD, is 83 points for the SCORAD, 103 points for the SCORAD Index and 72 points for the EASI. In addition, clinical studies often use the simpler IGA (Investigators’ Global Assessment), which usually uses a scale of AD severity from 0 to a maximum of 4 points. The guideline also recommends recording subjective aspects reported by patients using validated instruments [3]. In addition to itching and insomnia, this also includes recording other symptoms (e.g. POEM questionnaire) and quality of life (e.g. CDLQI/DLQI questionnaire). Complications of AD and extracutaneous comorbidity can also be taken into account when objectifying the severity of the disease [4].
Dupilumab and tralokinumab – no laboratory monitoring required
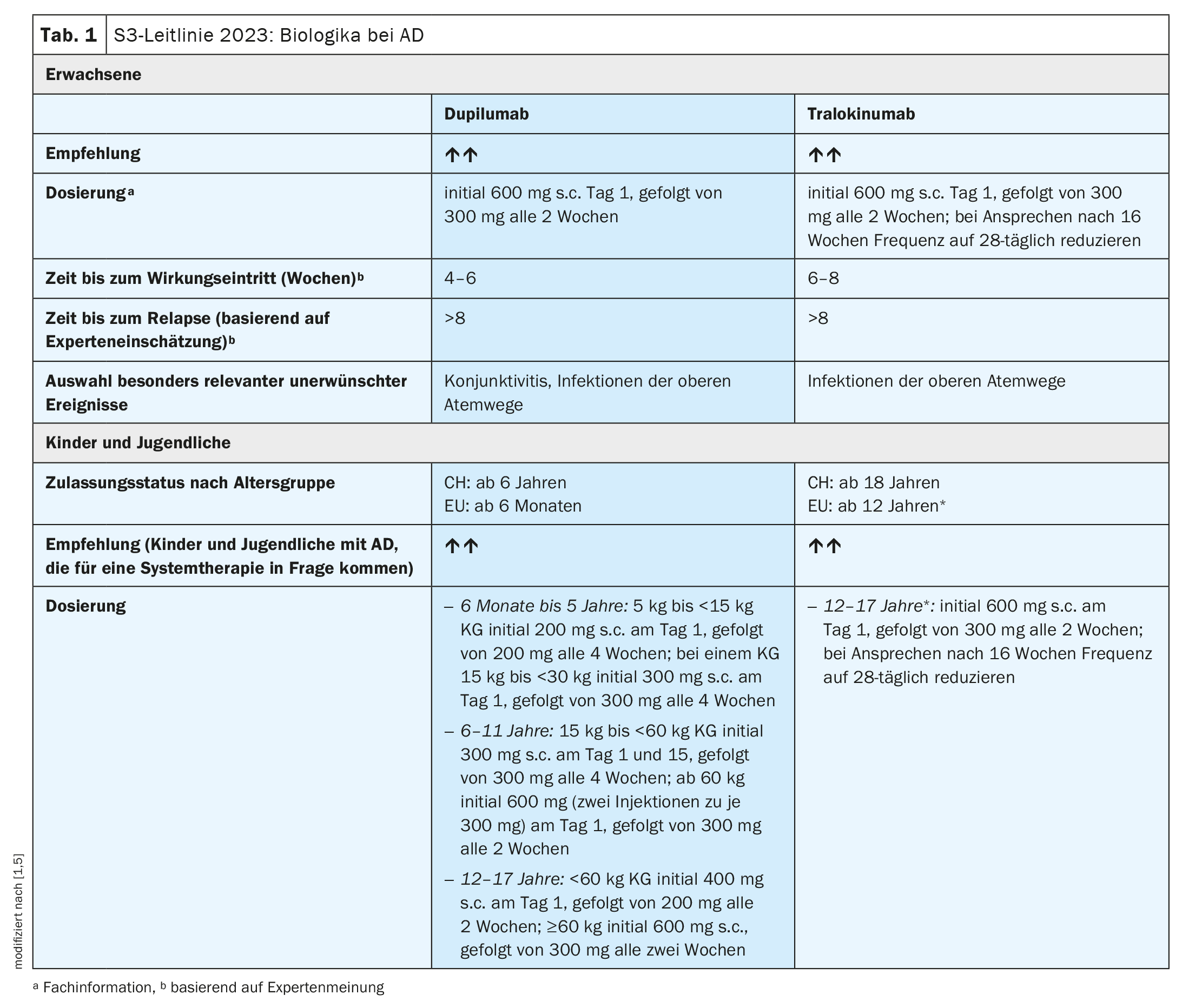
Both dupilumab, a monoclonal antibody (mAb) that inhibits interleukin (IL)-4 and IL-13 signaling, and tralokinumab – a mAb that neutralizes IL-13 – are recommended as long-term therapy for moderate to severe AD. In Switzerland, dupilumab is approved from the age of 6 and tralokinumab from the age of 18. Dupilumab should be preferred in patients with coexisting bronchial asthma, chronic rhinosinusitis**, prurigo nodularis or eosinophilic esophagitis, as specific evidence of efficacy is available in AD patients with these comorbidities.
** without/with nasal polyps
Dupilumab (Dupixent®): The approved dosage in adults consists of an initial subcutaneous dose of 600 mg, followed by maintenance doses of 300 mg every two weeks [1,5]. For children, dosage regimens are used depending on the age group and body weight (bw) (Table 1) .
Tralokinumab (Adtralza®): The dosage recommended in the guideline for adults and for adolescents aged 12 years and older is 300 mg every 2 weeks after an initial dose of 600 mg at the start of treatment [1]. In Switzerland, tralokinumab is currently only approved for use from the age of 18 [5]. The dosing frequency should be reduced from every 14 days to every 28 days after 16 weeks if there is a response to therapy [1]. In the further course, the dosing frequency (14 or 28 days) should be adapted to the clinical manifestation.
JAK inhibitors are rapidly effective – proper use is key
With JAK inhibitors, it is particularly important to clarify the individual risk of serious infections before starting treatment [1]. Use is not recommended in the case of a history of thromboembolic events or a genetically determined increased risk of thrombosis. The guideline points out that, according to a risk assessment procedure published by the European Commission in March 2023, JAK inhibitors should only be used in the following patient groups if no suitable treatment alternatives are available [1]: Age ≥65 years, known cardiovascular risk (e.g. heart attack or stroke), smokers (current or former long-term smokers), increased risk of cancer. Caution is advised in the presence of risk factors for blood clots in the lungs and deep veins (venous thromboembolism), even if the patients do not belong to the aforementioned risk groups. If JAK inhibitors are used in patients at risk of venous thromboembolism, cancer or severe cardiovascular problems, a reduced dose should be used.
Baricitinib (Olumiant®) is a selective JAK1 and JAK2 inhibitor for which a daily dose of 2 mg and 4 mg is recommended both in the guideline and according to the Swissmedic approval [1,5]. The guideline points out that treatment can be started at the higher dosage in patients under 65 years of age with severe AD after contraindications have been ruled out. AD patients with coexisting alopecia areata or comorbid rheumatoid arthritis benefit particularly from baricitinib, provided that the requirements for systemic therapy with JAK inhibitors are met.
Abrocitinib (Cibinqo®) is a selective JAK1 inhibitor that can be used in a daily dose of 100 mg or 200 mg according to the guideline [1]. It should be noted that therapy for severe AD can be started at the higher dosage in patients aged ≤64 years after contraindications have been excluded and the dosage should be adjusted to the clinical course after the response to therapy. According to the Swissmedic approval, however, the dosage recommendation is limited to 100 mg/day [5].
Upadacitinib (Rinvoq®) is also a selective JAK1 inhibitor. The guideline recommends doses of 15 mg and 30 mg daily [1]. As with the other two JAK inhibitors, it should be noted that upadacitinib can be started at the higher dose in severe AD after contraindications have been ruled out and the dose should be adjusted in the course of treatment if there is a response. According to the Swissmedic approval, however, a dosage recommendation is only available for 15 mg daily. AD patients suffering from concomitant diseases such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and axial spondyloarthritis or ulcerative colitis appear to benefit particularly from upadacitinib.
| The guideline, which was updated under the auspices of the German Dermatological Society, primarily aims to provide an evidence-based update on all aspects of care for patients with atopic dermatitis in order to provide doctors with the best possible support in their treatment. Numerous renowned experts from Germany, Austria and Switzerland were involved in drawing up the guidelines. As in previous versions, topical treatment options and systemic therapy are dealt with in separate chapters. The range of biologics and Janus kinase (JAK) inhibitors has been expanded since the last guideline update. In addition to the approved active substances, the guideline also mentions lebrikizumab, nemolizumab and omalizumab as promising drug candidates currently being researched for the systemic treatment of atopic dermatitis. |
| according to [1] |
Antipruritic therapy: combining measures as needed
Itching is a leading clinical symptom of AD and also represents a particular psychological challenge and burden [1]. The treatment of itching requires a multidimensional approach that addresses various factors, including dry skin, inflammatory processes, trigger factors, altered stimulus processing and neuroendocrine changes. The majority of therapeutic agents used in the treatment of AD are aimed at treating the inflammation and usually have an indirect effect on the pruritus. The itch-relieving effects of psychoeducational, stress-reducing and psychotherapeutic treatment approaches are described in the guideline in a separate chapter (“Psychoeducational, psychosocial and psychotherapeutic measures”) [1].
General aspects of disease management
The treatment of AD requires a variety of measures that must be individually tailored to the patient [1]. In addition to avoiding individual exacerbation factors, this includes individually adapted basic therapy as well as topical and/or systemic anti-inflammatory treatment. It is advisable to draw up an individual treatment plan. In general, a stage-adapted approach is recommended in order to adapt the therapy to the different individual phases depending on severity and chronicity. However, the step-by-step scheme only provides a guideline; the therapy should be individually adapted depending on age, course of the disease, localization and individual level of suffering.
While a number of innovations have been incorporated into the guideline with regard to modern systemic therapeutics from the biologics and JAK inhibitor drug groups, as mentioned above, the recommendations regarding conventional systemic agents have remained largely unchanged since the previous version. The more broadly effective, conventional immunosuppressants such as systemic glucocorticosteroids, ciclosporin, azathioprine, mycophenolate mofetil and methotrexate were used more frequently in the past for the systemic treatment of severe atopic dermatitis than they are today [1]. For conventional systemic therapeutics in particular, the ratio of expected benefit to risk should be individually assessed against the background of therapeutic alternatives [1]. With Ciclosporin, a systemic therapeutic agent that is still commonly used today, it is particularly important to ensure that blood pressure and renal function parameters are monitored closely.
Literature:
- S3 guideline “Atopic dermatitis” (AWMF register no. 013-027) 2023. Status: 16/06/2023, valid until: 15/06/2028.
- Schmitt J, et al.: Assessment of clinical signs of atopic dermatitis: A systematic review and recommendation. J Allergy Clin Immunol 2013; 132: 1337–1347.
- Schmitt J, et al.: Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012; 67: 1111–1117.
- Charman CR, Venn AJ, Williams HC: The patient-oriented eczema measure – Development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Archives of Dermatology 2004; 140: 1513–1519.
- Drug information, www.swissmedicinfo.ch,(last accessed 19.09.2023)
DERMATOLOGIE PRAXIS 2023; 33(5): 40–42