Rapid interdisciplinary consultation at the time of diagnosis of a malignancy is mandatory and enables “informed decision making” for those affected. Fertility protection should not worsen the prognosis. Failure to provide information may have liability consequences. In benign diseases of hematological, rheumatological and gynecological forms it is necessary to think about reduction of ovarian reserve. Even with moderate or small risk of ovarian damage, a shortening of the fertile life phase of up to ten years can be expected. After gonadotoxic therapy in childhood, the fertility reserve must be checked in the young woman. Cryopreservation of oocytes at this time may allow for later fulfillment of the desire to have a child.
According to the German Cancer Registry, 1800 children <and 30,000 adults aged 16-45 are diagnosed with cancer each year in Germany; in Switzerland, the number is around 250 children per year. With a survival rate of 80% for children and 50% for all ages, that’s thousands of survivors under 45 each year. If we add in the benign diseases with gonadotoxic therapy, the total number of people affected whose fertility is reduced because of a disease and the corresponding therapy is much higher. Evidence is now also available that pregnancy after cancer does not worsen prognosis, even in hormone-dependent tumors such as breast carcinoma. Fertility protection is therefore not an exotic topic but concerns oncologists, gynecologists, and family physicians [1]. The focus is on timely interdisciplinary consultation together with reproductive physicians in order to enable “informed decision making” for those affected – even in a life-threatening exceptional situation. Due to space constraints, I limit this paper to fertility protection in women.
Damage to the ovarian reserve
Surgery, chemotherapy and radiotherapy are the pillars of cancer therapy. Direct damage from the tumor itself or from surgical procedures involves malignancies in the small pelvis, i.e., colon, ovarian, uterine, and cervical cancers.
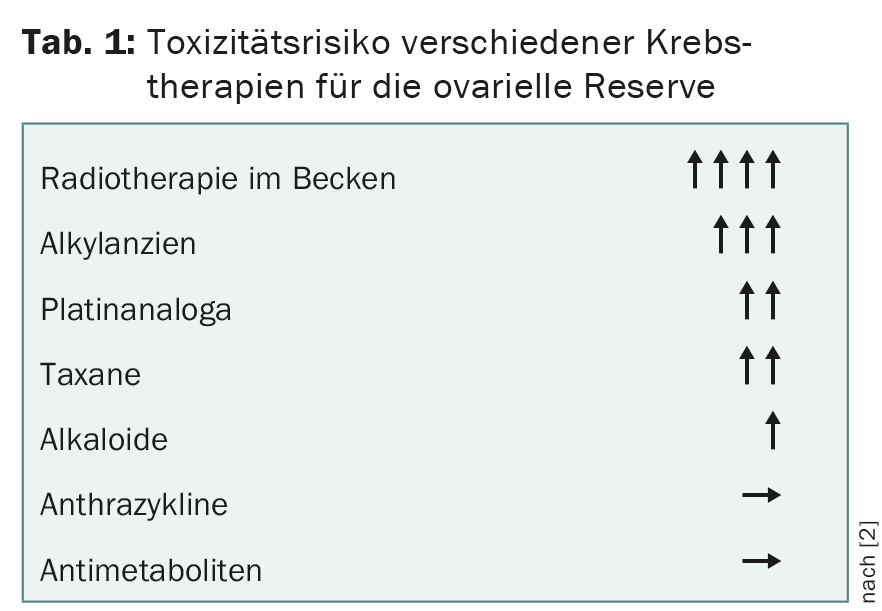
The gonadotoxic effect of chemotherapy depends on the substance, dose, and duration of therapy as well as the patient’s age and ovarian reserve (Table 1) [2]. An increased risk of miscarriage or malformation can be expected during the first six months after completion of chemotherapy. In contrast to chemotherapy, radiotherapy affects the ovaries and uterus [3].
90% of patients after total body irradiation before stem cell transplantation have ovarian insufficiency; after direct irradiation of the lesser pelvis, the figure is almost 100%. Consequential damage of childhood radiotherapy to the uterus includes fibrosis of the myometrium and atrophy of the endometrium with increased risk of miscarriage and prematurity. Transposition of the ovaries outside the irradiation field is not without problems.
Which method of fertility preservation can be offered depends on the oncological diagnosis and on the time window until the planned start of therapy (Tab. 2) . The vitrification technique of oocytes has decisively improved the chances of success. In parallel, cryopreservation of ovarian tissue with retransplantation has left the experimental stage. For both options, it is important to remember that tumor patients are often at higher risk for thromboembolism, bleeding, or infection. Appropriate precautions must be taken.

Cryopreservation of oocytes or zygotes
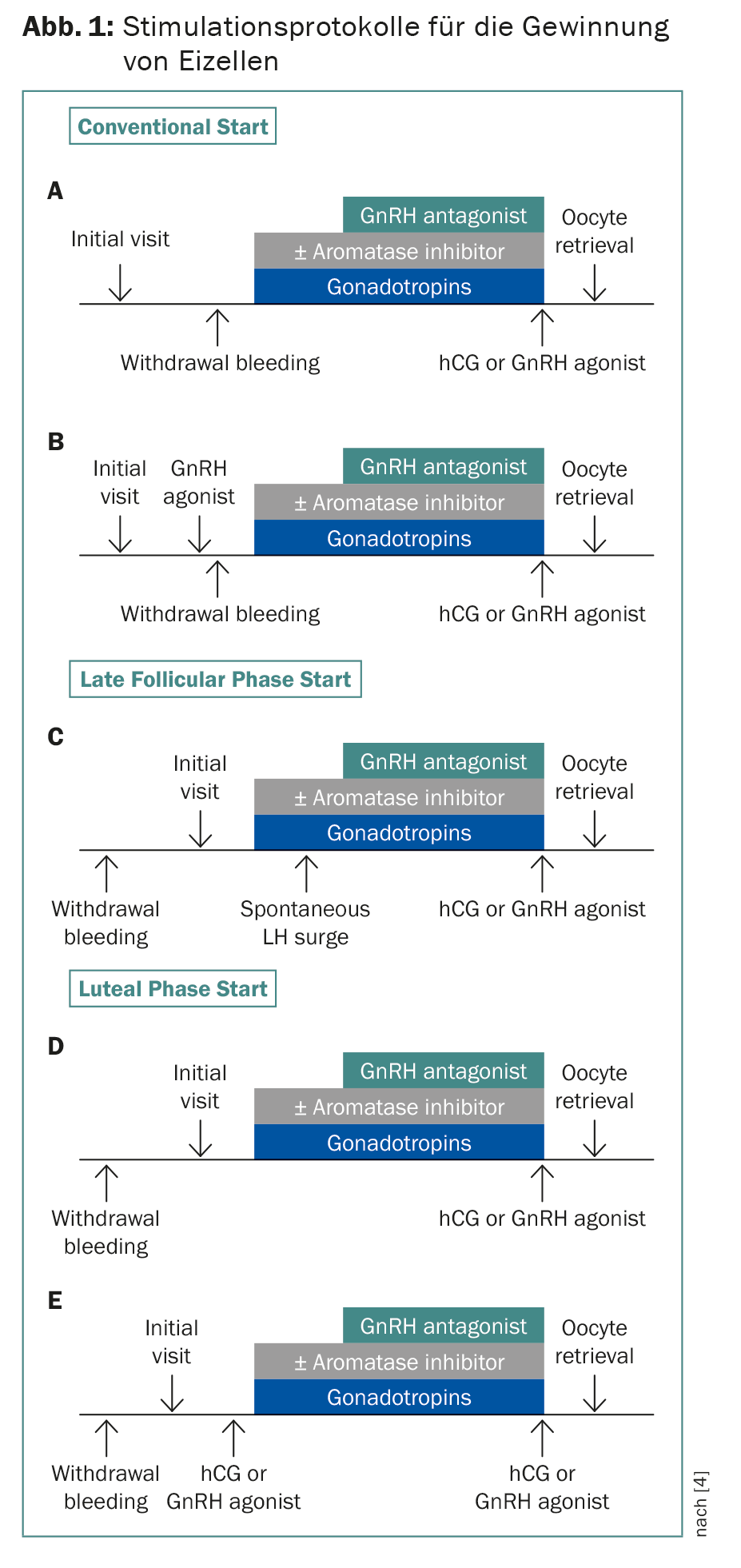
Cryopreservation of zygotes and embryos has been established for years. Thanks to vitrification technology, over 90% of oocytes now survive. Since time is usually of the essence, stimulation protocols have been developed that start independently of the onset of menstruation with a delay of two to three weeks for chemotherapy (Fig. 1) . Dual stimulations are also possible, starting in follicular and luteal phase. This allows twice as many eggs to be retrieved in four weeks. This is significant because in patients under 36 years of age, approximately 12-14 oocytes are needed for the subsequent birth of a child, and in patients aged 37-39 years, more than double that number [4]. The combination with letrozole lowers estrogen levels, which is important for hormone-dependent tumors and to prevent hyperstimulation syndrome [5]. In principle, I advise cryopreservation of oocytes rather than zygotes – even in a couple relationship – so that the woman retains reproductive autonomy.
Cryopreservation of ovarian tissue
As early as 2004, Donnez published an article about a birth after orthotopic transplantation of ovarian tissue [6]. In the meantime, the method has left the experimental stage. Laparoscopically, just under half of an ovary is removed and cryopreserved. In a retrospective analysis of the FertiPROTEKT network, 21 pregnancies were recorded after 95 transplants into the restovar or into a peritoneal pelvic pocket [7]. In 2011, the first Swiss baby was born after retransplantation of ovarian tissue by the team of the Kinderwunschzentrum Baden. For cryopreservation of ovarian tissue, a number of conditions must be met (Tab. 3).

GnRh analogues as adjuvant fertiprotective therapy.
The development of ovoprotective drugs is an urgent goal to enable patients to maintain natural fertility after cytotoxic therapy. Several compounds are under evaluation, but so far only GnRh analogues have become established. Suppression of gonadotropins to prepubertal levels and decreased utero-ovarian perfusion are postulated as protective mechanisms. A large, prospective randomized trial showed that the risk of premature ovarian failure can be reduced by 50% [8]. Concerns that therapy with GnRh analogues could reduce the effect of chemotherapy were allayed. The therapy can thus be generously recommended, even in combination with other methods.
Fertility protection before menarche
Today, more than 80% of all children who develop cancer in Switzerland survive. Two out of three suffer from late effects, including sterility. Of course, at the time of the cancer diagnosis, the thought of a ten-year-old girl’s later desire to have children is far away; it’s all about survival. For this very reason, interdisciplinary counseling of the parents and – as far as possible – of the affected children is indispensable, so that no important decision for “the afterlife” is missed. Euphorically, the first successful retransplantation after ovarian freezing in childhood was recently published [9]. Cryopreservation of ovarian tissue is therefore the only option for fertility protection in girls and very young women, associated with a surgical procedure with rare complications such as infections and bleeding.
Only 8% of girls with cancer are at high risk for posttherapeutic ovarian failure and thus benefit from ovarian tissue cryopreservation. However, even with low or medium risk, a shortened fertile period of up to ten years can be expected. This is all the more important because having children is increasingly being postponed until the 4th decennium. The average age of a woman at the birth of her first child in Switzerland is 31, and the trend is rising. As a consequence, all surviving girls in young adulthood need to be counseled regarding their ovarian reserve. The antimuller hormone and the number of antral follicles are determined. This is also possible under oral contraception, the values are then approx. 20% lower. A normal spontaneous cycle says little about a shortened fertile life phase. Cryopreservation of eggs at the beginning of 20 could leave the option open for the young woman to postpone the desire to have a child until the desired time – after training or studies.
Fertility protection in non-oncology patients.
Fertility protection is now well established in oncology patients. The situation is different for benign diseases that are treated with gonadotoxic substances (tab. 4) . It is also important to know that autoimmune diseases such as lupus erythematosus per se can be associated with reduced ovarian reserve [10]. In contrast to the acute situation when a malignancy is diagnosed, these patients usually have sufficient time for differentiated counseling and cryopreservation of a sufficient number of oocytes or zygotes before starting therapy.

What does the future hold?
Topics such as organ-preserving surgery for early-stage epithelial ovarian cancer, serous borderline tumors of the ovary or ovarian stem cell tumors, therapy with local and systemic progestin in early-stage endometrial cancer or even uterine transplantation after cervical cancer are under discussion and have already been tested in individual cases. In vitro maturation (IVM) of immature gametes is still experimental [11]. At present, many questions remain unanswered, which must be clarified by studies in order to be able to offer the patient an adequate and safe approach.
Legal aspects of fertility protection.
The Federal Law on Medically Assisted Reproduction explicitly allows storage of germ cells for an unlimited period of time in the case of gonadotoxic therapy. In malignancies, most affected individuals are informed about fertility-preserving methods; in benign processes, they are rarely informed before cytotoxic therapy. Winning liability lawsuits for failure to provide (or failure to document) counseling regarding sperm cryopreservation are already known. Unfortunately, costs for fertility protection are not covered by health insurance.
The consultation
Rapid interdisciplinary evaluation between oncologists and fertility specialists allows for differentiated counseling of affected individuals [12]. These include questions such as tumor type and stage, planned therapy with time window before start, risk of infertility after oncological therapy, general condition of the patient, but also psychological resilience, family situation and social environment. Under no circumstances should reproductive medical therapy jeopardize the patient’s prognosis. The discussion with the patient, possibly together with her partner or parents, is conducted according to a checklist (Tab. 5).

Clinical examination includes uterine and ovarian sonography (pelvic pathology, uterine and ovarian size, and number of antral follicles). The antimullerian hormone is currently considered the most important prognostic parameter with regard to ovarian reserve.
In addition to the technical details of a possible therapy, the consultation also focuses on the emotional aspects [12]. The patient and the whole environment are under the shock of a threatening diagnosis. Terms like danger of life, fear of death, loss, pain, suffering, grief and anger are in the room. By talking about fertility preservation, the desire to have children later, we can provide a counterpoint. We want to give space for hope, joy and meaning in life, for thoughts about life after cancer.
Literature:
- von Wolff M, et al: Fertility-preservation counseling and treatment for medical reasons: data from a multinational network of over 5000 women. Reprod Biomed Online 2015 Nov; 31(5): 605-612.
- Meirow D, et al: Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 2010; 53: 727-739.
- Mahajan N: Fertility preservation in female cancer patients: An overview. J Hum Reprod Sci 2015; 8(1): 3-13.
- Cakmak H, et al: Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril 2013; 100: 1673-1680.
- Rodriguez-Wallberg KA, et al: Fertility preservation and pregnancy in women with and without BRCA mutation-positive breast cancer. The Oncologist 2012; 17: 1409-1417.
- Donnez J, et al: Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364: 1405-1414.
- Van der Ven, et al: Ninety-five orthotopic transplantations of ovarian tissue after cytotoxic treatment in a fertility network – tissue activity, pregnancy and delivery rates. FertiPROTEKT 2015 (in preparation).
- Moore HC, et al: Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Eng J Med 2015; 372: 923-932.
- Demeestere I, et al: Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod 2015; 9: 2107-2109.
- Oktem O, et al: Ovarian function and reproductive outcomes of female patients with systemic lupus erythematosus and the strategies to preserve their fertility. Obstet Gynecol Surv 2015; 70(3): 196-210.
- Tomao F, et al: Special issues in fertility preservation for gynecologic malignancies. Crit Rev Oncol Hematol 2016 Jan; 97: 206-219.
- Baysal Ö, et al: Decision-making in female fertility preservation is balancing the expected burden of fertility preservation treatment and the wish to conceive. Hum Reprod 2015; 7: 1625-1634.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(1): 30-33.