The role of imaging in the diagnosis of PD currently lies mainly in the exclusion of causes of secondary parkinsonism. However, in the context of the clinic, it can also provide arguments for the presence of Parkinson’s disease or atypical parkinsonian syndromes. More recent developments aim to correctly diagnose individual patients using imaging in a clinical context.
Parkinson’s disease is the most common neurodegenerative movement disorder and the most common neurodegenerative disease after Alzheimer’s disease [1]. It was named after the English physician James Parkinson, who published the first detailed description of the clinical picture in 1817 [2].
The diagnosis of PD is made clinically, although it can only be definitively confirmed histopathologically. Usually, a combination of motor symptoms (rigor, tremor, bradykinesia) with asymmetric symptom onset, a response to L-dopa, a progressive course of more than ten years, and exclusion of other causes of parkinsonism are required [3, 4]. However, in early stages, not all symptoms are often present; moreover, other entities may cause overlapping symptomatology; postmortem studies have shown that a relatively high proportion of misdiagnoses of PD are made (76% confirmation at autopsy [5]).
Imaging methods (both morphologic and functional) may not aid in the diagnosis of PD by providing disease-specific signs, but may identify or support the diagnosis of other causes of PD. With the help of newer methods, attempts are also being made to establish the diagnosis of Parkinson’s disease itself for individual patients.
Cross-sectional imaging
The pathological-anatomical correlate of Parkinson’s disease is the loss of dopaminergic cells in the substantia nigra pars compacta (Fig. 1), which, however, is rarely reflected by a narrowing of the same in MRI. In most cases, only nonspecific atrophy with dilation of the internal and external CSF spaces is found. At present, the real value of imaging lies in its ability to rule out other, potentially treatable, causes of symptomatology.
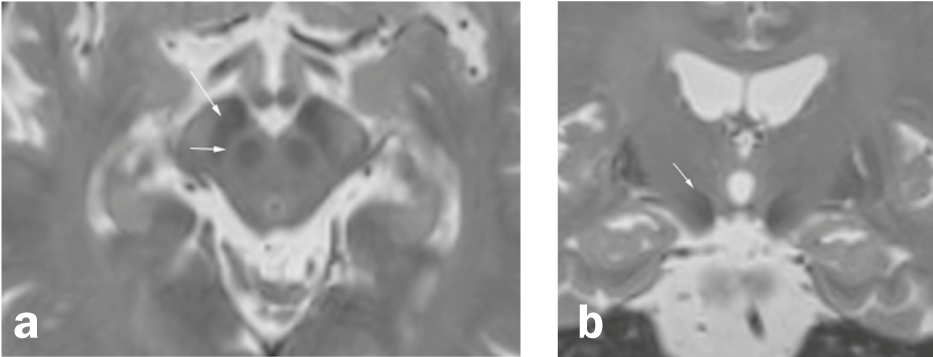
Fig. 1 Substantia nigra and surroundings
Axial (a) and coronary (b) Reconstruction of a high-resolution 3D T2-weighted sequence (MRI). (a) The substantia nigra, located between the nucleus ruber and the fiber bundles of the crus cerebri, consists of two layers, a hypointense zone in the posterior portion of the crus cerebri (pars reticularis; long arrow) and a relatively hyperintense layer (short arrow) between the pars reticularis and the nucleus ruber (although the zones in T2 weighting do not correspond exactly to the anatomical localization). (b) The subthalamic nucleus (arrow) is a nucleus medial to the internal capsule and superolateral to the ncl. ruber and one of the target areas of deep brain stimulation in Parkinson’s disease.
Exclusion of other causes of parkinsonism: Differential diagnoses of PD include secondary parkinsonism of toxic, metabolic, or vascular etiology and pseudoparkinsonism, e.g., due to normal pressure hydrocephalus [6] or chronic subdural hematoma, some of which are easily detected by CT and/or MRI ( Fig. 2).
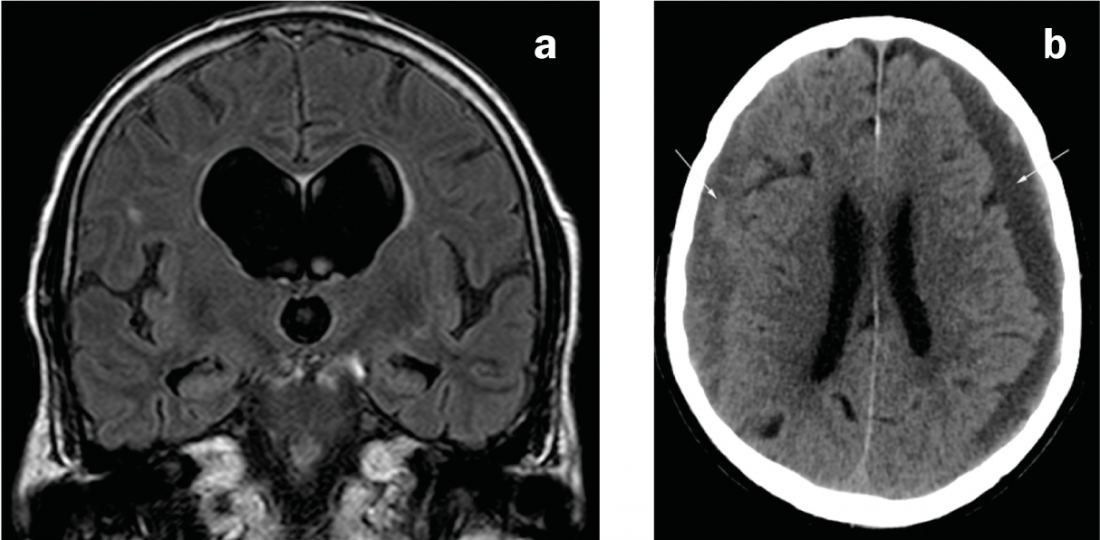
Fig. 2: Causes of secondary parkinsonism
(a) Coronary MRI in FLAIR weighting. Disproportionately dilated ventricular system compared to the external CSF spaces in normal pressure hydrocephalus. b) CT axial. Bilateral subdural hematomas of mixed age: left predominantly hypodense (i.e., chronic), right predominantly isodense (i.e., subacute).
Exclusion of atypical parkinsonian syndrome: Differential diagnoses that are more difficult clinically and radiologically include the atypical parkinsonian syndromes MSA (multisystem atrophy), PSP (progressive supranuclear gaze palsy), and CBD (corticobasal ganglionic degeneration). Imaging manifestations can often only be detected at advanced stages.
Signs of MSA-C (cerebellar type) include atrophy of the pons and cerebellum (including brachium pontis) and the “hot cross bun” sign (cross-shaped hyperintensity in the pons on axial T2/FLAIR-weighted recordings due to degeneration of pontine neurons and pontocerebellar connections) (Fig. 3a and b). Typical sign of MSA-P (putaminal/Parkinsonian type, a differential diagnosis to Parkinson’s disease due to extrapyramidal symptoms) is hypointensity of the dorsolateral putamen in T2-/T2*-weighted sequences with adjacent hyperintense stripe (“putaminal slit sign” due to a putaminal volume loss) (Fig. 3c). The described findings can be found in all subtypes of MSA, although infratentorial findings are more common in MSA-C than in MSA-P (and putaminal findings, in turn, are more commonly described in MSA-P) [7].
In PSP, there is atrophy of the mesencephalon (including the superior colliculi) and the superior pedunculus cerebellaris, which leads to the “penguin” sign on sagittal images (flat mesencephalon and preserved roundness of the pons are thought to lead to the resemblance to the silhouette of a penguin with a small head and round belly) (Figs. 3d and e). The periaqueductal gray may appear T2 hyperintense.
Findings of CBD include a narrowing of the precentral and postcentral gyrus (Fig. 3f), subcortical gliosis with T2/FLAIR hyperintensity and prominent parasagittal atrophy. Atrophy of the basal ganglia may be discrete.
Because all of these signs can be very subtle and there is some overlap in the findings of all of the aforementioned clinical pictures, various measurements (e.g., diameter and area of the mesencephalon and pons or pedunculus cerebellaris superior and medius) and indices were used in an attempt to define objective signs for differential diagnosis of the various entities and thus to make specific diagnoses beyond group-level differences. For example, the “MR-parkinsonian-index” [8] allows to distinguish a possible or probable PSP of PD, MSA and normal controls.
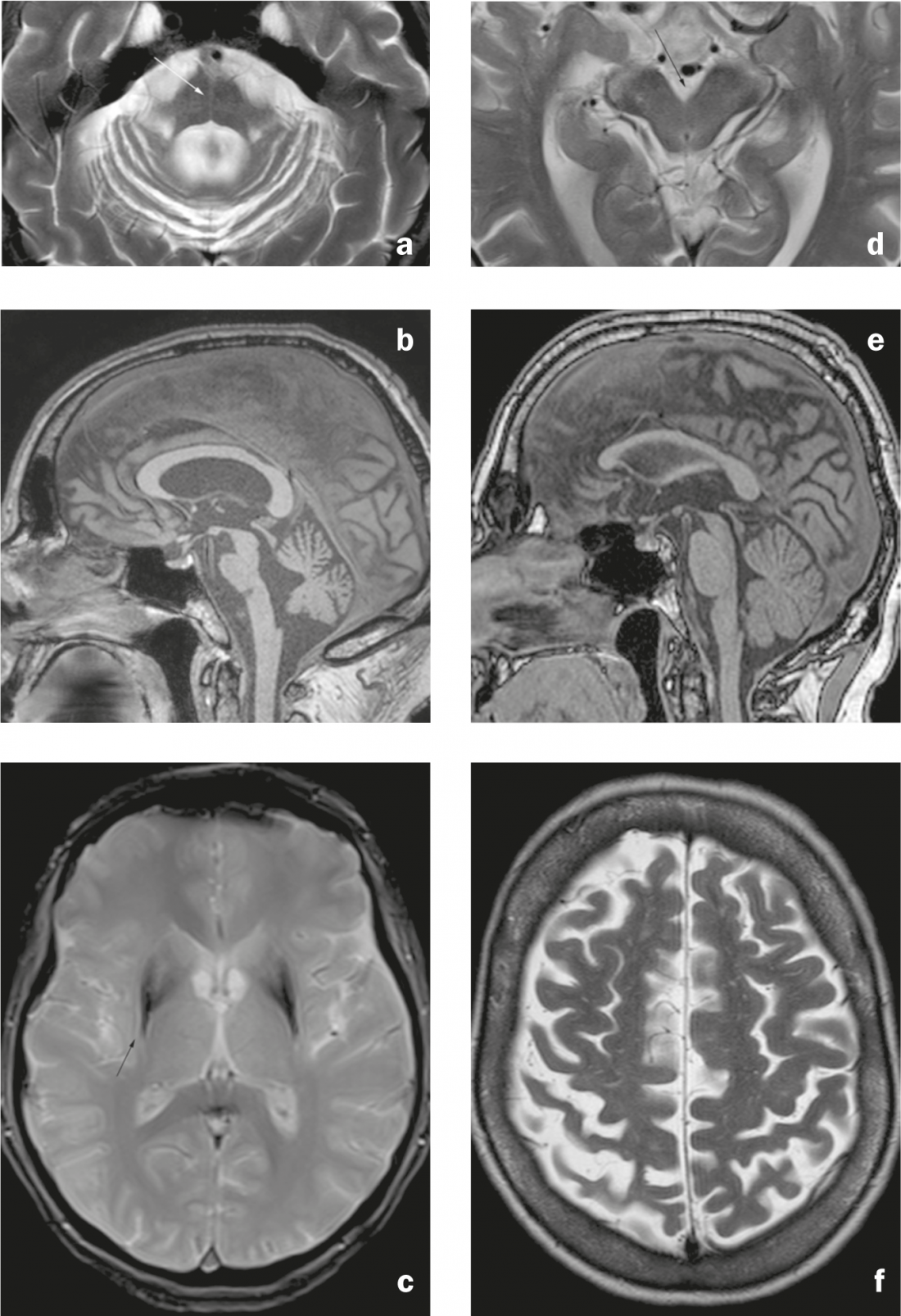
Fig. 3: Atypical Parkinson’s syndromes. MSA-C: (a) Axial T2-weighted image. Pontine and cerebellar atrophy with “hot cross bun” sign (arrow), dilatation of the fourth ventricle, and narrowing of the brachium pontis. b) Sagittal image in T1 weighting. Flattening of the pons. MSA-P: c) Axial T2*-weighted image. Hypointensity of the dorsolateral putamen with adjacent linear hyperintensity (arrow). PSP: (d) Axial T2-weighted image. Atrophy of the mesencephalon with dilation of the interpeduncular cistern. e) Sagittal T1-weighted image. Atrophy of the mesencephalon, which appears narrow compared to the pons (“penguin” or “hummingbird” sign). CBD: f) Axial T2-weighted image. Atrophy of precentral gyrus and postcentral gyrus with dilatation of central sulcus (right accent).
New developments in MR imaging: in addition to conventional imaging, advanced MRI techniques such as “diffusion weighted imaging” (DWI), “diffusion tensor imaging” (DTI) or susceptibility weighted sequences such as T2* or “susceptibility weighted imaging” (SWI) are also being investigated with regard to the differentiation of PD patients and patients with atypical syndromes [9, 10]. New sequences or new variants of existing sequences will be used to detect subtle changes in the very small anatomical structures affected in PD at earlier stages. For example, using the PADRE technique (“phase difference enhanced imaging”; realized on a 3T MRI), blurring of the boundary between substantia nigra and the fiber bundles of the crus cerebri has been demonstrated in Parkinson’s disease patients [11], which is not detectable on conventional MR sequences.
In addition, with the aid of advanced structural analysis techniques of gray (“voxel based morphometry”, VBM) and white (“tract based spatial statistics”, TBSS) matter [12, 13], attempts are being made to detect small signal differences on MRI that are not apparent by purely visual analysis.
Preoperative imaging: In addition to ruling out causes of parkinsonism other than PD, cross-sectional imaging is helpful for preoperative targeting (globus pallidus and especially subthalamic nclus) before implantation of deep brain stimulation electrodes.
Nuclear medicine methods
Nuclear medicine methods allow the detection of a disturbance of the dopaminergic system and are used in clinical practice as an additional diagnostic test in unclear cases. Thus, using different ligands in SPECT or PET, the availability of presynaptic dopamine transporters (DaT) or the activity of dopa decaroboxylase can be investigated. In particular, the “DaT scan” (the [123I]FP-CIT-SPECT) has gained widespread use as a test for unclear Parkinson’s syndromes. Parkinson’s disease shows a reduction in ligand binding(Fig. 4), which allows it to be distinguished from essential tremor (but not with certainty from atypical Parkinson’s syndromes) [14].
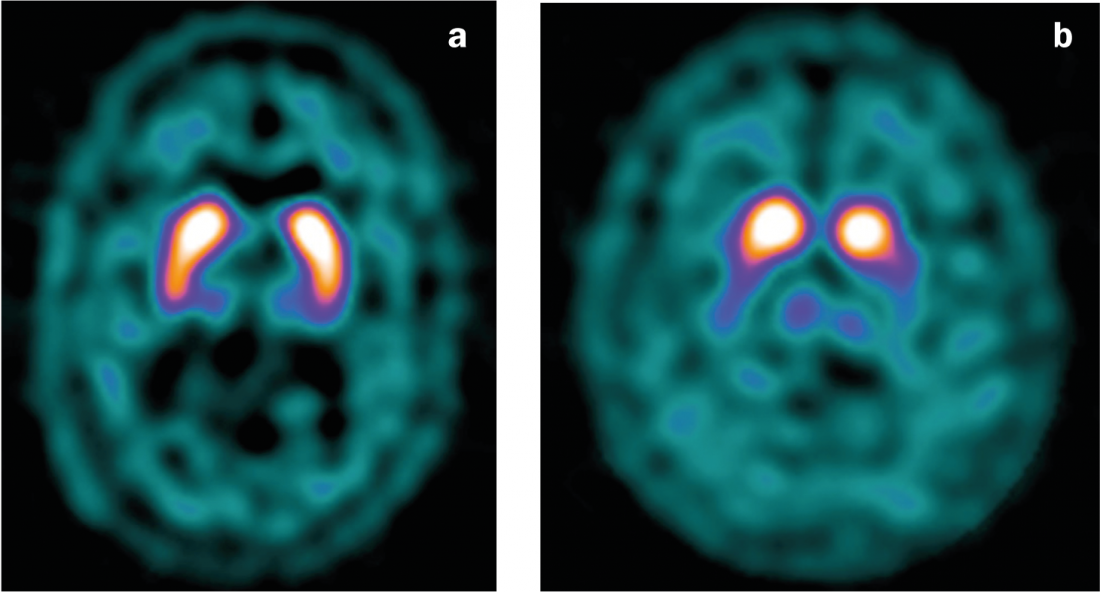
Fig. 4: DaT scan
(a) Normal findings with symmetrical binding of the ligand in the striatum.
(b) Decreased ligand binding in PD, particularly left-sided (with predominantly right-sided clinic).
Classification methods
In addition to the morphologic analyses described above, classification analyses are increasingly being undertaken to improve diagnosis in individual patients. Unlike group studies, these are not based on the idea of comparing patient groups with healthy control subjects to detect disease-related structural changes [15], whose differences may be significant at the group level but not pronounced enough at the individual level to allow diagnosis in clinical practice. Rather, they pursue the goal of identifying or correctly classifying individual patients. Classification studies are performed using “support vector machine” analyses of various data (e.g., DTI or SWI sequences). A “support vector machine” classifier is an analysis method that aims to classify data into two or more groups (e.g., Parkinson’s disease/atypical Parkinsonism) based on pattern recognition. For this purpose, in a first step, processed images (i.e., transferred to a standard space, for example) are analyzed for the most discriminative features using various algorithms. In a second step, the determined classifier is tested for sensitivity, specificity and accuracy using a new set of data [16]. Preliminary results of the analysis of DTI and SWI data showed high classification accuracy for patients with PD (vs. patients with atypical PD symptoms [12, 13]).
Literature list at the publisher
Sven Haller, MD
Isabelle Barnaure, MD