DORIS (Definition of Remission in SLE) and LLDAS (Lupus Low Disease Activity State) are established measures of remission in systemic lupus erythematosus (SLE), but their feasibility and validity have not yet been investigated in patients with moderate or high disease activity and severity. Greek researchers have now taken on this task.
DORIS or LLDAS are associated with an improvement in health-related quality of life, which has also been confirmed in randomized controlled trials (RCTs). Based on these findings, the European Alliance of Associations for Rheumatology (EULAR) has recommended that SLE therapy should be designed with the aim of achieving both parameters.
However, the feasibility and generalizability of the treatment goals in different clinical scenarios have proven to be problematic: With few exceptions, previous studies have examined the LLDAS and DORIS definitions in unselected SLE cohorts without explicit criteria for disease actiAvity. This may have led to a bias in the results, as patients with a milder disease course are more likely to reach the target values and have better long-term outcomes overall.
Dr. Sofia Pitsigavdaki from the Department of Rheumatology and Clinical Immunology at the University of Crete School of Medicine in Heraklion, Greece, and colleagues conducted a study that included SLE patients with moderate to severe disease activity who were followed at multiple consecutive visits over an average of five years [1]. In addition to analyzing the achievement of remission (DORIS) and low disease activity (LLDAS) – overlap was possible – several methods were used at each presentation and cumulatively over time to determine the impact on the development of damage and severe disease flares.
Focus on moderate to severe SLE
The work focused on moderate or severe SLE requiring intensification of therapy, as it is not clear whether the existing definitions of remission/LLDAS are feasible and effective in this underrepresented patient group in previous studies. These patients usually start with higher disease activity and are exposed to more glucocorticoids, making it more difficult to achieve the recommended goals while increasing the risk of harm.
In a cohort of 348 patients, DORIS and LLDAS were achieved at least once during follow-up by 215 (61.8%) and 323 (92.8%) individuals, respectively. The median time to first occurrence of DORIS was 15 months, the corresponding estimate for LLDAS was 9 months. A total of 97 (27.9%) and 193 (55.5%) patients spent ≥24 months in DORIS and LLDAS, respectively.
These results show that both targets are achievable in patients with active moderate-to-severe SLE, with LLDAS having a higher feasibility than DORIS.
In fact, of 1577 LLDAS visits, 771 (48.9%) met the DORIS definition (LLDAS+/DORIS+), while the remaining 806 (51.1%) did not meet this definition (LLDAS+/DORIS-).
This suggests that LLDAS partially overlaps with DORIS and a proportion of patients may fall into a certain state of low or minimal – but not zero – disease activity, the authors said.
Patients with higher SLE activity are usually treated with more glucocorticoids, and they tend to develop organ damage and relapses more frequently.
Therefore, the scientists investigated whether the existing targets were protective in moderate to severe disease.
In an analysis of individual visits, both DORIS and LLDAS were associated with a reduced risk of new organ damage (HR 0.64; 95% CI 0.42-0.97 and HR 0.63, respectively; 95% CI 0.46-0.89) and severe disease relapses (HR 0.34; 95% CI 0.22-0.51 and HR 0.39, respectively; 95% CI 0.29-0.51) at the next visit.
Both early and late achievement of DORIS and LLDAS were associated with a reduction in future organ damage and severe disease relapses, supporting the validity and generalizability of the parameters in moderate/severe SLE, the researchers point out.
LLDAS and DORIS overlapped in about 50% of visits, which is less than the overlap of LLDAS and DORIS shown in previous studies – possibly due to differences in patient characteristics and duration of follow-up. Therefore, it remains uncertain whether LLDAS exerts an additional protective effect beyond remission.
Risk of organ damage and severe relapses reduced
The result of their evaluation that LLDAS+/DORIS visits had a significantly lower risk of later development of organ damage and severe relapses seems plausible given the linear association of SLEDAI and glucocorticoid use with the risk of adverse outcomes such as organ damage.
It supports the recommendation to consider remission as the preferred treatment goal, with low disease activity being a good alternative.
The protective effect of goal achievement against organ damage and severe disease relapses was particularly pronounced in patients with persistent DORIS and LLDAS for at least 6 months.
Studies with different settings and cohorts have shown that the shortest duration in the target range associated with a reduction in damage progression can range from 3 months for LLDAS to at least 2 consecutive years for DORIS and LLDAS.
These results underscore the importance of prolonged disease stabilization with appropriate treatment modifications (if needed) to ensure optimal long-term outcomes in SLE.
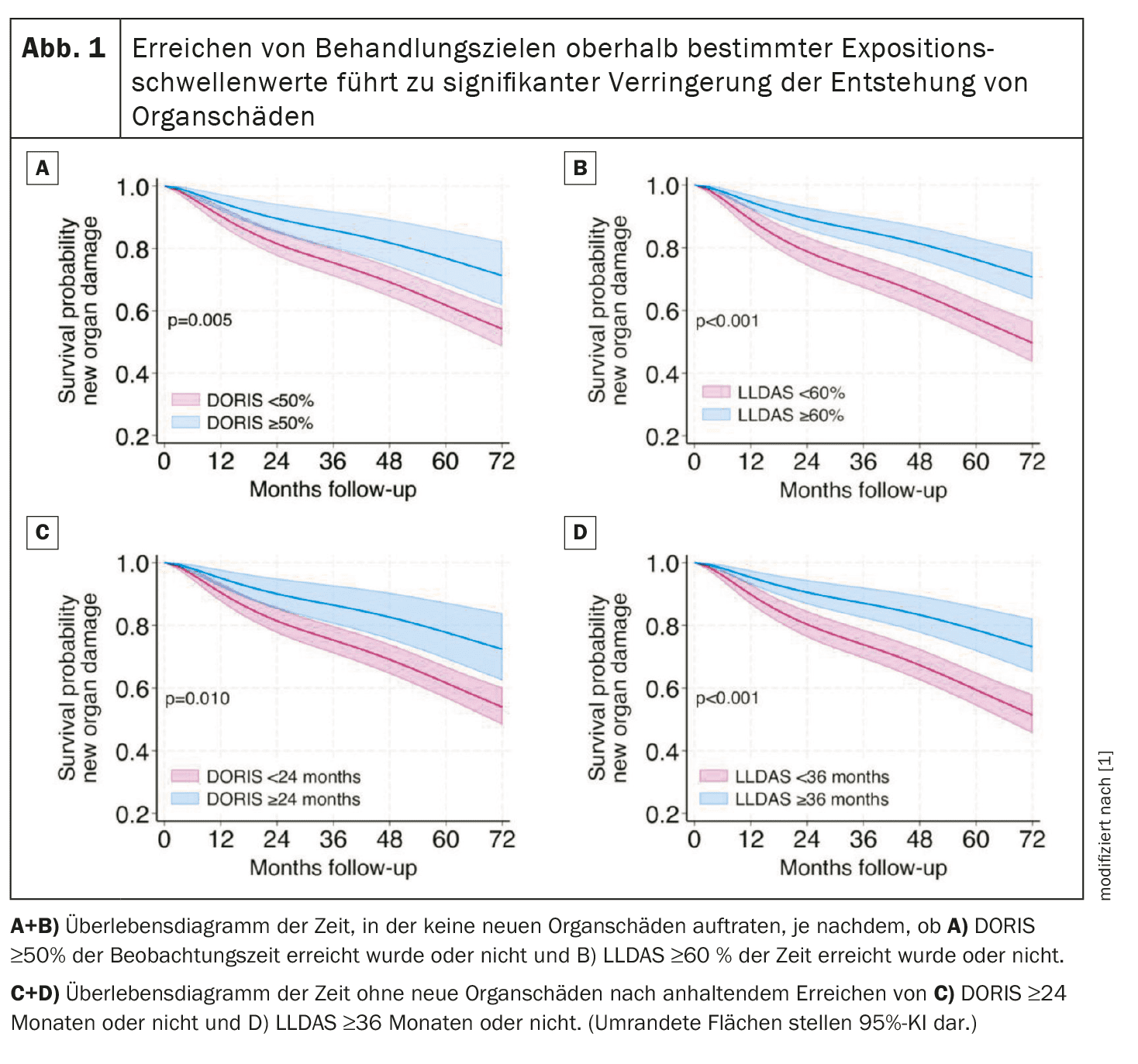
DORIS ≥50% and LLDAS ≥60% of cumulative time (respectively ≥24 and ≥36 consecutive months) showed the best combination of prevalence/sensitivity and specificity for a damage-free course (Fig. 1).
Dr. Pitsigavdaki and colleagues reviewed the impact of exposure-defined DORIS and LLDAS targets by documenting their association with a lower incidence of adverse events, associated hospitalizations and deaths.
This confirms previous reports in SLE showing that prolonged achievement of the two targets can reduce mortality.
The association between DORIS or LLDAS and risk of adverse events and/or hospitalization is novel and reminiscent of similar protective effects of remission and low disease activity in rheumatoid arthritis.
Furthermore, the observation of lower DORIS/LLDAS rates (with concomitant increase in disease flares) in patients with prevalent mucocutaneous and joint disease is consistent with previous studies in white patients and merits further discussion.
Although these particular manifestations may be inherently difficult to treat, they were more commonly treated with methotrexate, azathioprine and to a lesser extent leflunomide, belimumab or rituximab.
The authors point out that these associations are not proof of causality and that the choice of some drugs may have been influenced by physician and patient preferences or intolerances.
The lower frequency of DORIS/LLDAS could also be related to the SLEDAI instrument, which requires the complete disappearance of arthritis, rash, etc. for the corresponding items to be scored 0.
However, the removal of the mucosal and arthritis items from the SLEDAI instrument would not have significantly altered Dr. Pitsigavdaki’s results.
Take-Home-Messages
- Remission (DORIS) and low disease activity (LLDAS) are realistic targets in active moderate-to-severe SLE, associated with a reduction in irreversible organ damage and severe disease relapses, emphasizing their value in clinical care.
- At least 6 months of sustained DORIS/LLDAS is sufficient for protection; at the individual level, longer achievement of these targets (at least 24 months) has high specificity (>80%) for a harm-free prognosis and protects against several other adverse outcomes, suggesting that they may be useful for treat-to-target strategies and clinical trial design.
- Achieving targets above specific observation time thresholds shows high specificity for favorable prognosis, including significantly lower rates of adverse events and hospitalizations, potentially impacting treat-to-target implementation and clinical trial design.
- In lupus arthritis and mucosal diseases, which are predominantly treated with conventional agents, there is an increased propensity for disease flare-up and low attainment of treatment goals, highlighting the potential benefits of novel targeted agents.
Literature:
- Pitsigavdaki S, et al: Rheumatology and Clinical Immunology, University of Crete School of Medicine, Heraklion, Greece, Pragmatic targets for moderate/severe SLE and their implications for clinical care and trial design: sustained DORIS or LLDAS for at least 6 months is sufficient while their attainment for at least 24 months ensures high specificity for damage-free progression. Annals of the Rheumatic Diseases 2024; 83: 464-474; doi: 10.1136/ard-2023-224919.
InFo RHEUMATOLOGIE 2024; 6(1): 26-27