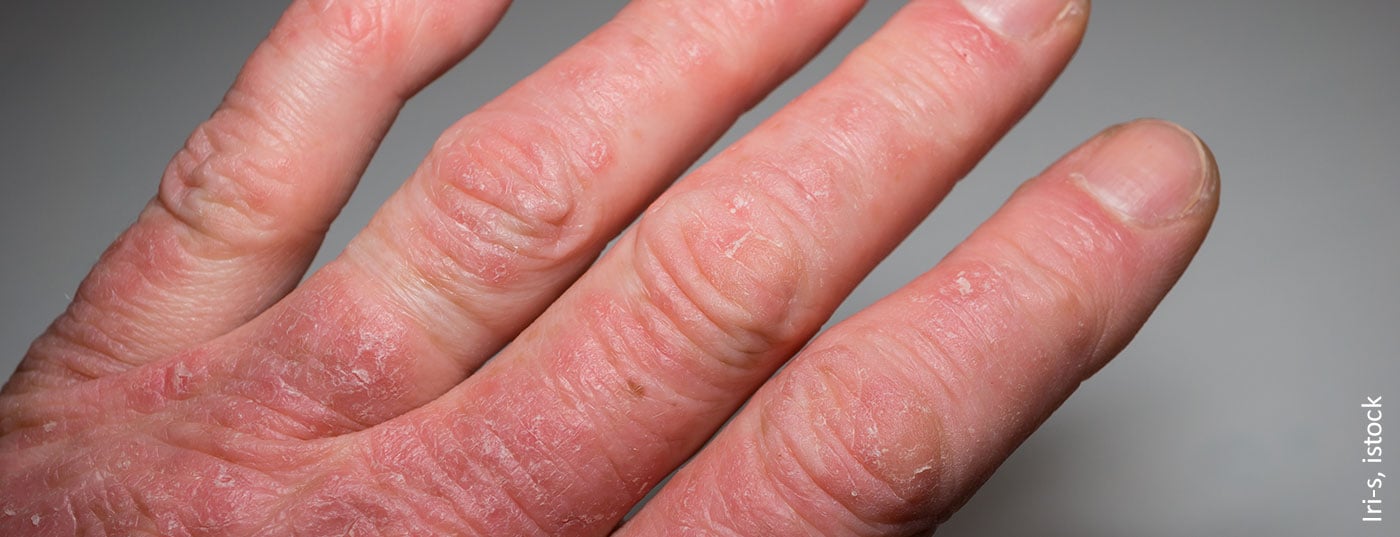
Since psoriatic arthritis often occurs years after the onset of skin psoriasis, dermatologic evaluation of incipient joint involvement is particularly important. Early detection of joint involvement has important therapeutic implications. Through the use of modern targeted therapies, a much higher degree of disease control can be achieved than was the case in the past.
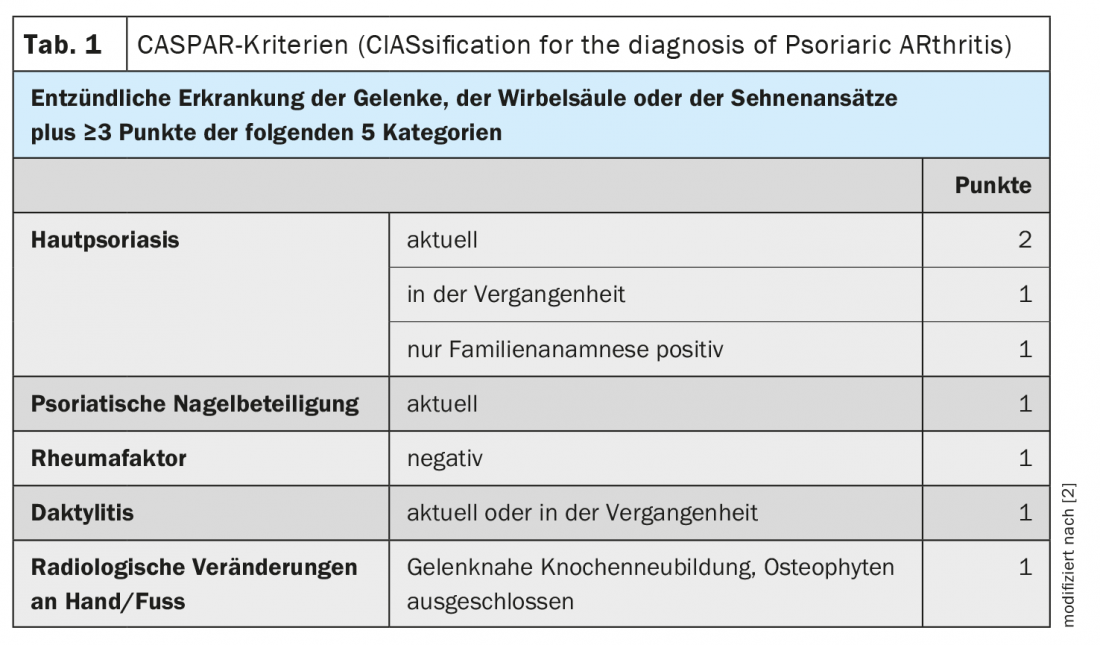
The CASPAR (Classification Criteria for Psoriatic Arthritis) criteria are available to narrow down whether psoriatic arthritis (PsA) or other joint symptoms are present in psoriasis patients with joint symptoms (Table 1) [1,2]. According to this classification system, the main criterion for PsA is the presence of inflammatory changes in the joints, spine or tendons or tendon insertions. In addition, at least three other scores must be obtained to confirm the PsA diagnosis with a sensitivity of 91.4% and a specificity of 98.7% [3]. These criteria include a current or anamnestic skin psoriasis, a positive family history (in one or more first- or second-degree relatives), psoriatic nail changes as well as a diagnosed dactylitis, the absence of rheumatoid factor in the serum, and radiologically detectable changes in the area of the hands or feet (bone neoplasms near the joints) (Table 1) [3].
Screen PsA at-risk patients for joint involvement in a timely manner
Screening tools such as the GEPARD questionnaire (box) are helpful in everyday practice to pre-select patients more easily and to initiate an interdisciplinary/rheumatologic evaluation. “It is very important to make the diagnosis early because, on the one hand, joint destruction can occur early and, on the other hand, we know that psoriatic arthritis can be linked to other diseases/comorbidities that also need to be treated and taken into account,” said Prof. Uwe Wollina, MD, Chief Physician, Department of Dermatology and Allergology, Dresden Municipal Hospital [4]. Adequate therapy initiated in time can counteract a progressive course and the associated physical and psychosocial problems. If patients with skin psoriasis not only have nail involvement, but environmental stress factors/psychosocial stressors are present, the risk of developing PsA is increased, Prof. Wollina said. Patients at risk should be monitored to avoid missing preclinical or clinical signs of incipient PsA.

Differential diagnostic considerations: Is it really PsA?
“The most important differential diagnosis from the rheumatologic field is rheumatoid arthritis,” said the speaker [4]. Rheumatoid arthritis (RA) predominantly affects women and is characterized by specific features that clinically distinguish it from PsA. These include morning stiffness, which is much more pronounced in RA than in PsA. And besides, “Arthritis in more than 3 joints is much more common in rheumatoid arthritis,” Prof. Wollina said. Arthritis in the hand, or rheumatoid nodules, rheumatoid factor, and loss of bone mass near the joint are all classic signs suggestive of RA. In contrast, a characteristic feature of PsA is involvement of distal finger/toe joints, as well as dactylitis and enthesitis. “We have new bone formation near the joint – this is a very late sign, but it is a characteristic one,” said Prof. Wollina [4].
Another differential diagnosis to PsA is osteoarthritis, which, unlike PsA, is not associated with either dactylitis or enthesitis. Furthermore, it should be remembered that elevated uric acid levels can occur not only in gout but also in PsA [4]. This is partly due to increased production and partly due to reduced excretion of uric acid [4]. One explanation for increased uric acid production is that psoriatic skin has a faster metabolism, so there is increased nucleic acid breakdown. There are different explanations for reduced uric acid excretion depending on the respective comorbidities of the affected psoriasis patients (e.g. obesity, IBD).
GRAPPA recommends domain-specific treatment for psoriatic arthritis
In general, therapy should be tailored to individual patient characteristics and aligned with the severity of the respective disease manifestations of PsA. This is reflected in the treatment algorithm proposed by the GRAPPA expert group (The Group for Research and Assessmentof Psoriasis and Psoriatic Arthritis) (Fig. 1) [5]. If conventional disease-modifying agents (csDMARDs) are not effective, several biologics (bDMARDs) and synthetic agents (tsDMARDs) are available. According to the GRAPPA recommendations, an important criterion is which of the following disease domains predominates: If this is peripheral arthritis, both bDMARD and tsDMARD may be considered if there is an inadequate response to csDMARD. In particular, there is a good evidence base for TNF-alpha-i and for the groups of IL-12/23-i, IL-17-i, and IL-23-i. In PsA patients with predominant axial involvement, TNF-alpha inhibitors and IL-17-i, as well as JAK-i, have been particularly useful, in addition to NSAIDs. In PsA patients with predominant enthesitis and dactylitis, the best evidence is for TNF-alpha-i as well as IL-12/23-i, IL-17-i, and IL-23-i. And in nail psoriasis, the best results are obtained with TNF-alpha-i, IL-12/23-i, IL-17-i, and IL-23-i, as well as with PDE-4-i. If psoriatic skin involvement is predominant and csDMARDs do not prove effective, a broad spectrum of drug groups is available (Fig. 1): TNF-alpha-i, IL12/23-i, IL-23-i, IL-17-i, JAK-i, PDE-4-i.
Comorbidities should also be considered in the treatment decision. In PsA patients with inflammatory bowel disease (IBD), the preferred use of TNF-i (except etanercept), IL-12/23-i, and JAK-i is advised. Patient preferences regarding the form of application and treatment intervals are also taken into account in the decision.
In Switzerland, in addition to various TNF-alpha-i, the following bDMARDs are currently approved for the treatment of PsA: IL17-i (secukinumab, ixekizumab); IL-23-i (guselkumab, risankizumab); IL-12/23-i (ustekinumab). Among tsDMARDs, the PDE-4-i apremilast and the JAK-i upadacitinib and tofacitinib are available [7].
Congress: World Psoriasis Day
Literature:
- Meissner, T: Orthop Rheuma 2018; 21: 10.
- Taylor W, et al: CASPAR Study Group. Arthritis Rheum 2006; 54(8): 2665-2673.
- Ziupa E-M: Prevalence of psoriatic arthritis in dermatologic patients with psoriasis, Inaugural Dissertation, 2016, https://publikationen.uni-tuebingen.de,(last accessed 11/18/2022).
- “Latest news on psoriasis disease”, World Psoriasis Day 2022, Deutscher Psoriasis Bund and Hautnetz Leipzig, Oct. 29, 2022.
- Coates, LC et al: GRAPPA Treatment Recommendations 2021, eEULAR 2021, Abstract OP0229, http://dx.doi.org/10.1136/annrheumdis-2021-eular.4091
- Härle P, et al: Z Rheumatol 2009; 69(2): 157-163.
- Drug information: www.swissmedicinfo.ch,(last accessed 11/21/2022).