Among the most common birth defects are congenital heart defects. With the help of modern developments in cardiac surgery, these can usually be successfully corrected. However, this is not the end of the story. This is because adult heart defect patients are at increased risk of cardiovascular complications. For example, infective endocarditis is a growing threat.
Congenital heart defects are the most common birth defects. Approximately 1/100 live births are affected and about 6/1000 babies have a complex congenital heart defect [1]. Without intervention, many of these heart defects have a very poor prognosis. However, thanks to development of modern cardiac medicine, especially cardiac surgery, most of these defects can be successfully repaired nowadays and the majority of affected patients now reach adulthood [2]. This trend is causing the cohorts of adults with congenital heart defects to grow rapidly [3,4]. Mended is not healed! Although most adult heart defect patients have a good quality of life, the risk for cardiovascular complications is high. In addition to a high risk of cardiac arrhythmias and an increased risk of developing heart failure, infective endocarditis is a growing threat.
Epidemiology and risk of endocarditis
In population-based studies, a slow but steady increase in the incidence of infective endocarditis has been observed over the past two decades, particularly in the elderly [5]. This increase is likely due to the aging of society and increased cardiac interventions in the elderly population. These interventions include implantation of transvenous pacemakers and defibrillators, and in particular increased valve interventions due to the availability of percutaneous catheter-based treatment options.
In contrast, the incidence of infective endocarditis in the cohort of adults with congenital heart defects is several times higher than in the general population. In contemporary population-based studies, the incidence of infective endocarditis in the general population is approximately 7.6 cases/100,000 patient-years, whereas the incidence in adults with congenital heart disease is 133 cases/100,000 patient-years [5–7]. Accordingly, the number of affected adults with infective endocarditis in congenital heart disease requiring treatment at specialized centers is rising steeply (Fig. 1).
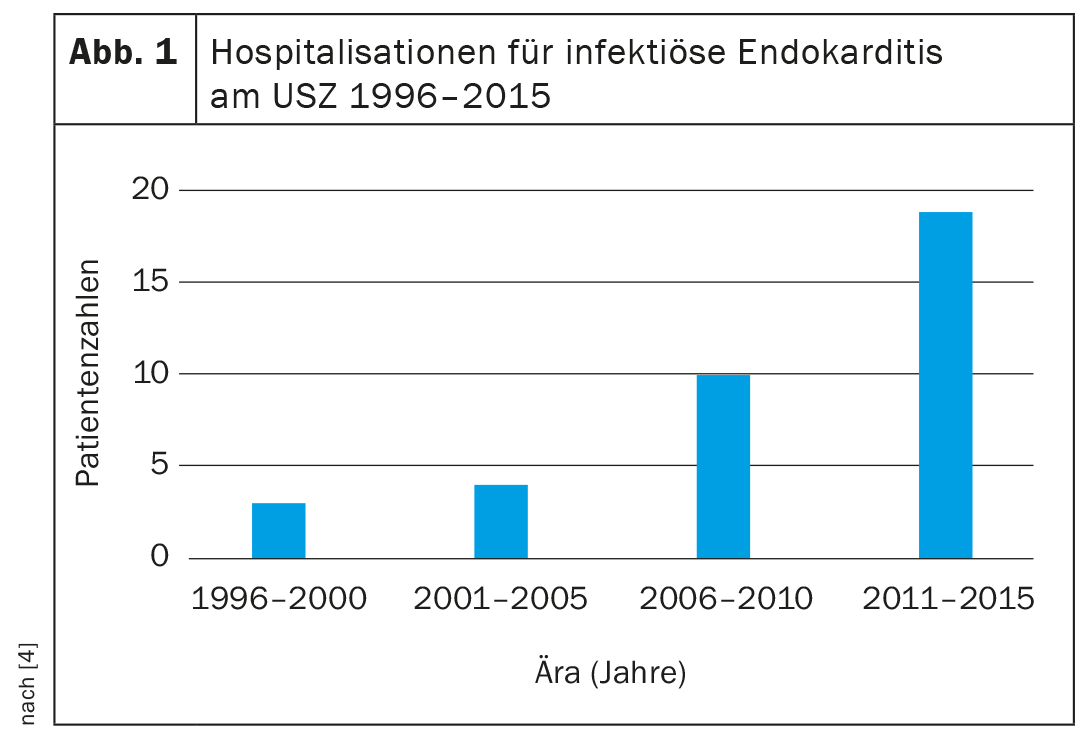
The reason for the increase in the number of cases and increased risk of endocarditis in adults with congenital heart defects is, on the one hand, the rapid increase in the number of affected patients and, on the other hand, the fact that about 20-25% of affected adults with congenital heart defects belong to the group with the highest risk of infective endocarditis (Overview 1) [4,8].

Presentation and diagnosis
As in patients with infective endocarditis in acquired heart disease, the diagnosis of infective endocarditis in adults with congenital heart disease is based on the patient’s clinical presentation, individual cardiopathy, evidence of the causative agent (in tissues, blood cultures, or serologies), and evidence of endocarditis-typical morphologic changes on heart valves or foreign material (pacemaker or ICD electrodes). The clinical diagnostic criteria for infective endocarditis are summarized in review 2 (modified Duke criteria) [9].
Special features of adults with congenital heart defects
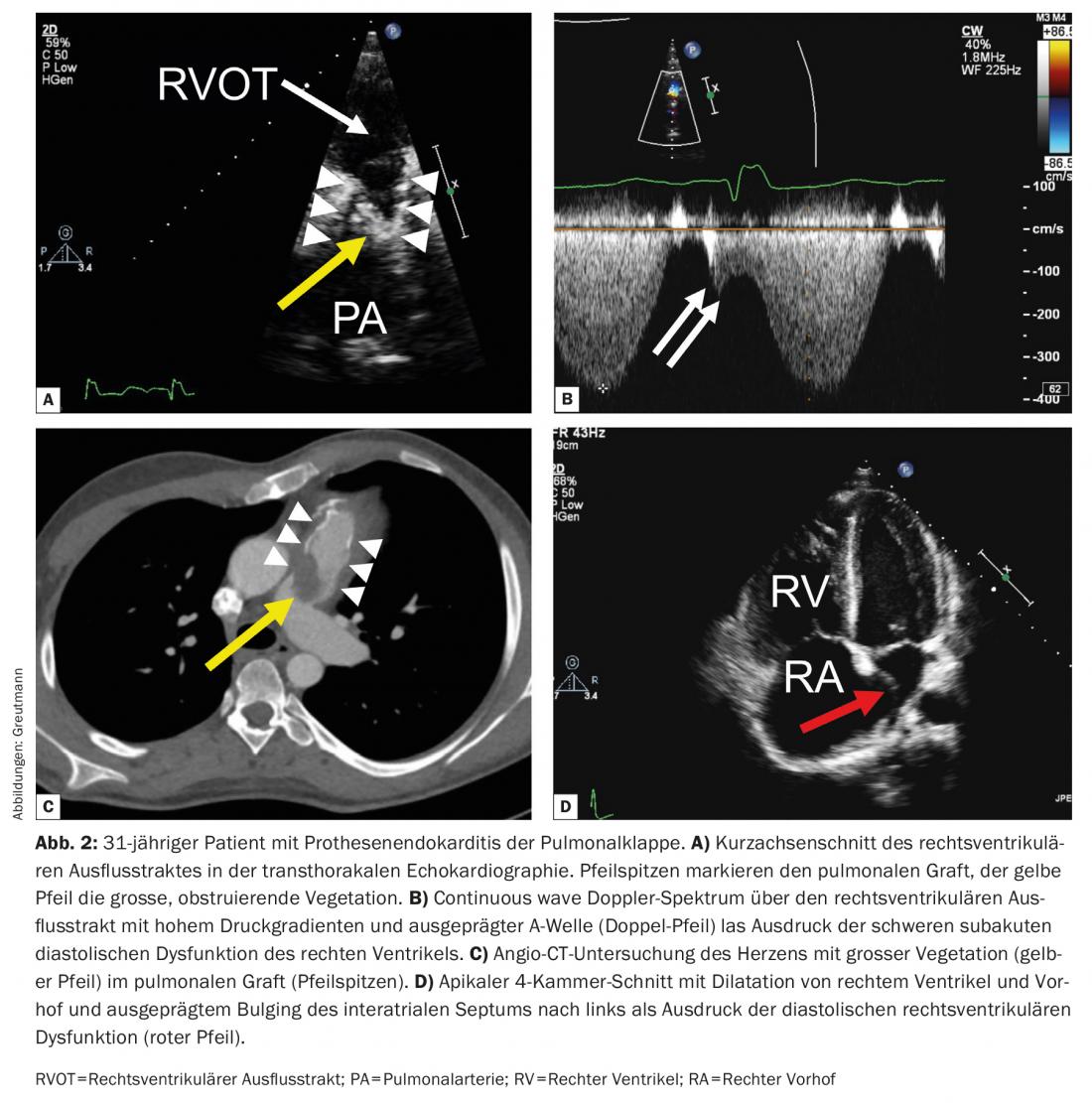
Pulmonary valve endocarditis: Pulmonary valve prosthesis endocarditis illustrated in the case vignette presents a special challenge. Residual right ventricular outflow tract hemodynamics are common in adults with repaired congenital heart defects. In particular, the popularity of Ross surgery and the liberal indication for secondary pulmonary valve replacement in patients with residual pulmonary valve regurgitation after repair surgery in childhood, has led to a rapidly growing cohort of adolescents and younger adults with pulmonary valve prostheses [10]. In cases of degeneration of the pulmonary valve prosthesis, percutaneous pulmonary valve replacement has become the standard procedure over the last two decades. Several studies have shown that the risk of infective endocarditis after prosthetic pulmonary valve replacement, especially after percutaneous valve replacement, is very high and amounts to at least 2% per patient year [11].
While pulmonary valve endocarditis is an exceptional rarity in the general population, pulmonary valve prosthetic endocarditis is now the most common form of endocarditis in adults with congenital heart defects. Presentation, clinical signs, and imaging are often atypical and unfamiliar to non-specialist cardiologists and primary care physicians. In our experience, therefore, diagnosis is often delayed, which is associated with increased morbidity [12].
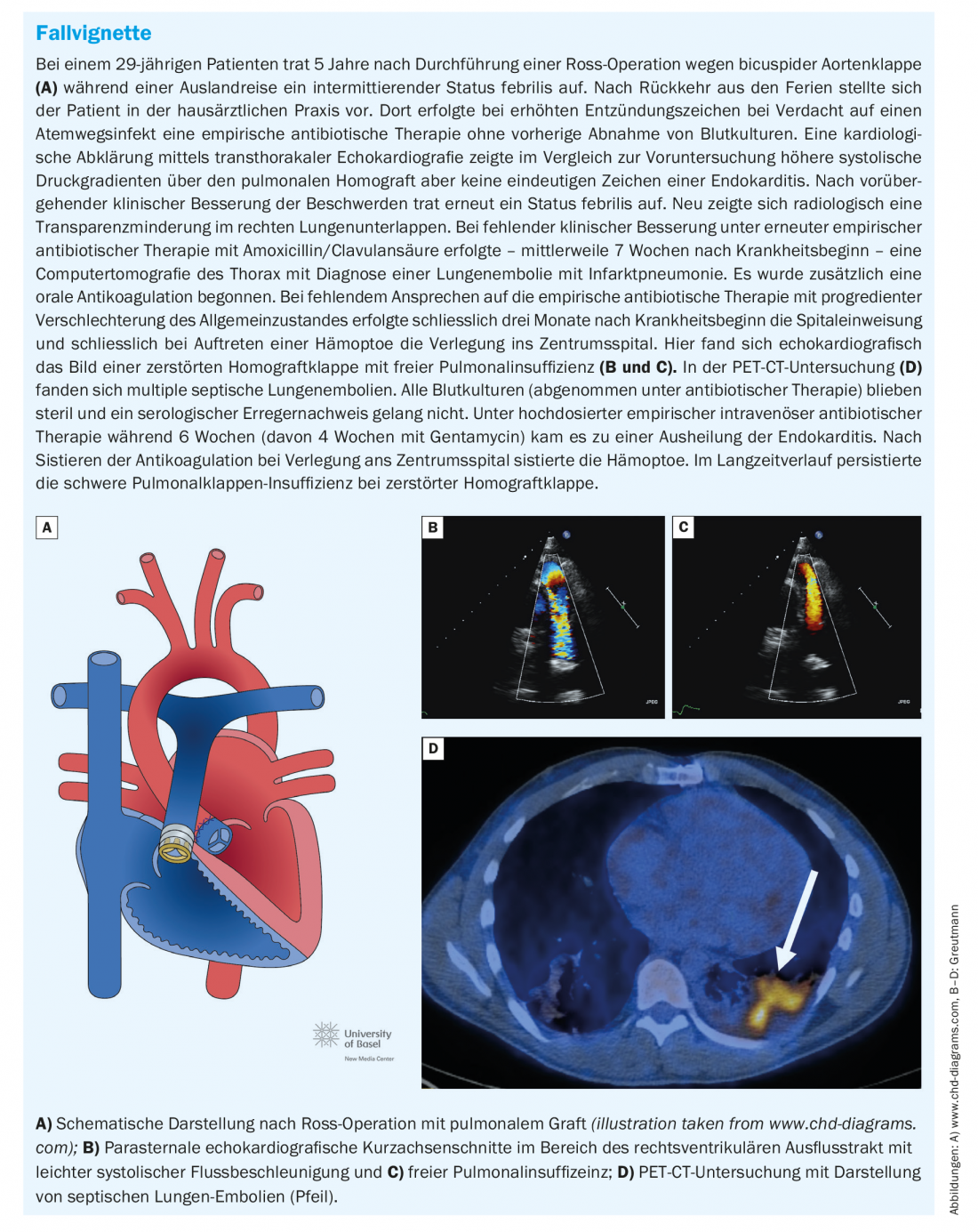
Unlike native valve endocarditis, which often presents with progressive valve regurgitation, pulmonary valve prosthetic endocarditis most commonly presents with valve obstruction as the main hemodynamic manifestation (approximately 60% of cases). This can be very pronounced and can lead to cardiogenic shock. Apart from uncontrolled infection, severe valve obstruction is the most common indication for emergency surgical valve replacement. Early transfer of affected patients to a specialized center with the possibility of rapid surgical intervention is therefore important. Due to the anterior location of the pulmonary valve prosthesis in the thorax, they are sometimes difficult to visualize on tranesophageal echocardiography, and a careful transthoracic examination should always be performed primarily. Septic pulmonary emboli are common and may be helpful as an additional minor criterion in making the diagnosis when the prosthetic valve is difficult to visualize echocardiographically. Clinically manifest septic pulmonary embolisms or even lung abscesses occur frequently with delayed diagnosis and must be differentiated from simple pneumonia if the constellation is appropriate (Fig. 2) [12].
Endocarditis in the area of jet lesions
In the pathogenesis of endocarditis, we assume that (subclinical) endocardial defects are the most important risk factor for the development of endocarditis due to everyday bacteremia. These endocardial defects result from turbulent blood flow at abnormally functioning heart valves or endovascular foreign bodies. High blood flow velocities at high pressure gradients favor these endocardial defects (left-sided heart valves, small ventricular septal defects), whereas structures with lower flow velocities are hardly (e.g., native pulmonary valves in patients without pulmonary hypertension) or never affected by endocarditis (e.g., atrial septal defects).
However, endocardial defects can also occur in the area of jet lesions and cause endocarditis in the area of cardiac structures that are not primarily altered. This particularly concerns insufficient bicuspid aortic valves and ventricular septal defects. Bicuspid aortic valve often results in eccentric aortic regurgitation directed to the anterior mitral leaflet. Fig. 3A-C) shows a typical example of mitral valve endocarditis in primary aortic valve pathology. Endocarditis in small ventricular septal defects also usually occurs in the area where the systolic jet of the left-to-right shunt meets the subvalvular apparatus of the tricuspid valve (Figure 3D-F).

Prevention, prophylaxis and patient education
Until recently, the focus of endocarditis prevention was to identify patients at highest risk for endocarditis (Overview 2) and instruct them regarding antibiotic shielding for dental procedures (with appropriate endocarditis identification). However, as illustrated in the case vignette, in our clinical experience, delayed diagnosis of endocarditis often occurs with sometimes devastating consequences. This is due to the fact that endocarditis caused by oral germs is responsible for only about a quarter of all endocarditis. In addition, studies show that transient bacteremia with oral germs occurs several times a day, for example, when brushing teeth or chewing [13]. Therefore, antibiotic prophylaxis during dental procedures is of secondary importance for the prevention of infective endocarditis. This aspect is taken into account by the revised Swiss endocarditis guidelines [14]. The focus is now primarily on patient education for all patients at increased risk of endocarditis. In collaboration with the Swiss Heart Foundation, information brochures have been developed that can be given to appropriate patients at risk of endocarditis. These flyers can be ordered free of charge from the Swiss Heart Foundation (www.endocarditis.ch).
Our own experience shows that with a simple patient education program, which we have implemented in our daily consultation routine for several years, the delay from symp-tom onset to diagnosis and correct therapy of infective endocarditis can be significantly reduced [12].
The most important preventive measure to avoid pulmonary valve prosthesis endocarditis is to avoid implanting a prosthesis! Therefore, we are challenged to very carefully weigh the benefits and risks for the long-term outcome when determining the indication for pulmonary valve replacement.
Literature:
- Hoffman JI, Kaplan S: The incidence of congenital heart disease. Journal of the American College of Cardiology 2002; 39(12): 1890-1900.
- Moons P, Bovijn L, Budts W, et al: Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010; 122(22): 2264-2272.
- Marelli AJ, Ionescu-Ittu R, Mackie AS, et al: Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014; 130(9): 749-756.
- Padrutt M, Bracher I, Bonassin F, et al: Impact of growing cohorts of adults with congenital heart disease on clinical workload: a 20-year experience at a tertiary care center. Swiss medical weekly 2017; 147: w14443.
- Erichsen P, Gislason GH, Bruun NE: The increasing incidence of infective endocarditis in Denmark, 1994-2011. Eur J Intern Med 2016; 35: 95-99.
- Toyoda N, Chikwe J, Itagaki S, et al: Trends in infective endocarditis in California and New York State, 1998-2013. Jama 2017; 317(16): 1652-1660.
- Kuijpers JM, Koolbergen DR, Groenink M, et al: Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. European heart journal 2017; 38(26): 2048-2056.
- Tobler D, Schwerzmann M, Bouchardy J, et al: Swiss Adult Congenital HEart disease Registry (SACHER) – rationale, design and first results. Swiss medical weekly 2017; 147: w14519.
- Habib G, Lancellotti P, Antunes MJ, et al: 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). European heart journal 2015;36(44): 3075-3128.
- O’Byrne ML, Glatz AC, Mercer-Rosa L, et al: Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. The American journal of cardiology 2015; 115(1): 118-124.
- Van Dijck I, Budts W, Cools B, et al: Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart 2015; 101(10): 788-793.
- Babic D, Hammerli R, Santos Lopes B, et al: Impact of a structured patient education program on early diagnosis of prosthetic pulmonary valve endocarditis. Cardiology in the young 2021: 1-6.
- Lockhart PB, Brennan MT, Sasser HC, et al: Bacteremia associated with toothbrushing and dental extraction. Circulation 2008; 117(24): 3118-3125.
- Sendi P, Hasse B, Frank M, et al: Infective endocarditis: prevention and antibiotic prophylaxis. Swiss medical weekly 2021; 151: w20473.
CARDIOVASC 2022; 21(1): 6-10