Nowadays, there is a wide range of treatment options for androgenetic alopecia (AGA). Topical preparations with the active ingredient minoxidil and the oral 5-α-reductase inhibitors finasteride and dutasteride are among the classic evidence-based therapeutic measures. However, several studies are now also available on low-level laser therapy and platelet-rich plasma (PRP).
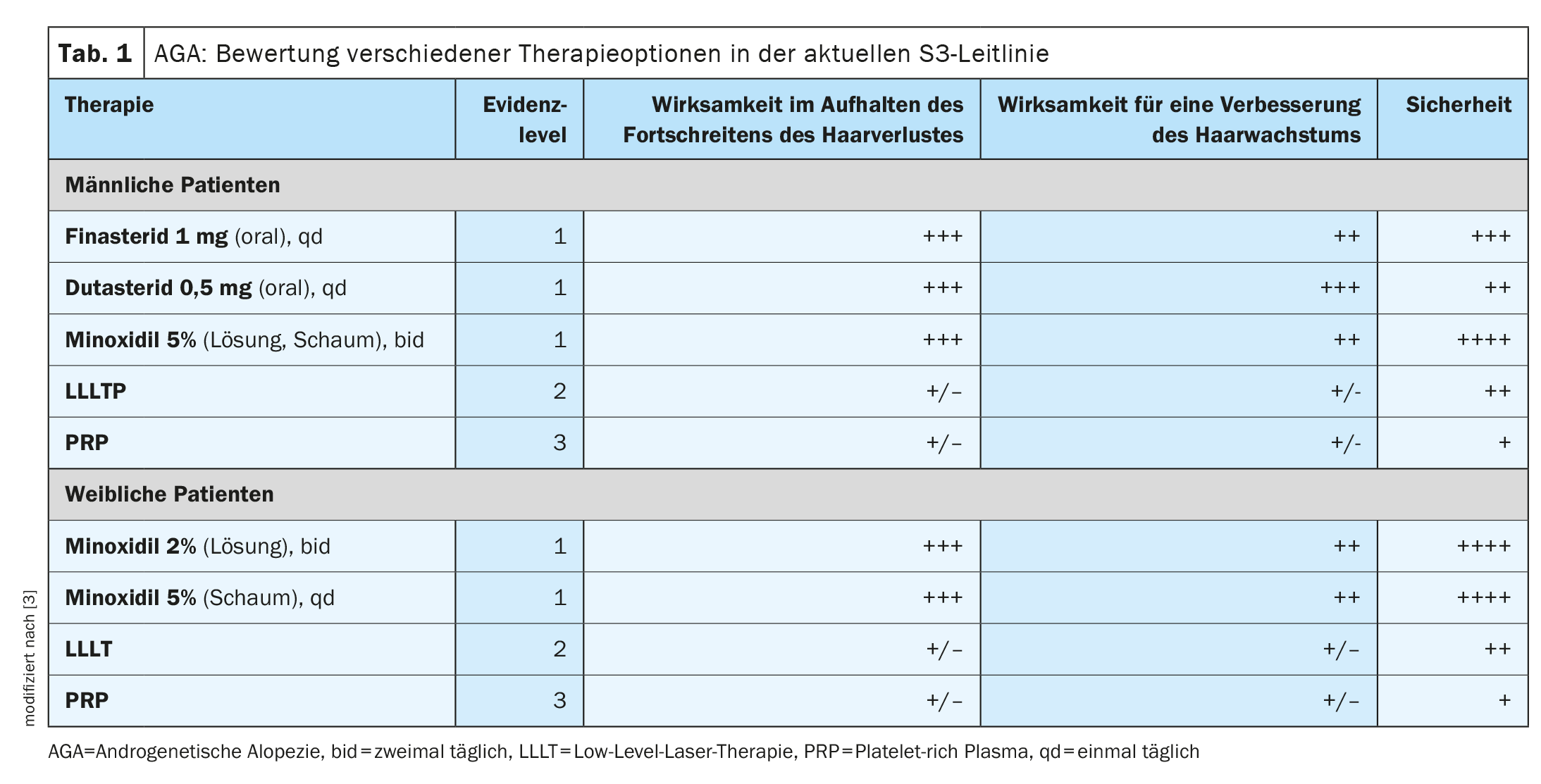
Hair loss is a symptom that greatly changes the appearance and can significantly affect the quality of life. In both men and women, around 95% of all cases of hair loss are caused by androgenetic alopecia (alopecia androgenetica, AGA). This congenital hair loss (box) is therefore the most common cause of alopecia in both sexes [1,4–7]. Kaiser et al. have summarized the current evidence on various treatment options for AGA in a review article published in 2023 [2]. And the S3 guideline for the treatment of AGA in men and women published in JEADV in 2018 is based on the evaluation of current therapies based on the analysis of 47 scientific articles that meet the inclusion criteria according to AGREE standards [3]. The assessment criteria included effectiveness in halting the progression of hair loss, effectiveness in improving hair growth and the safety of the respective treatment option (Table 1) .
Topical minoxidil: sulfotransferase activity in the hair follicle is relevant
Minoxidil is approved for the topical treatment of the scalp in androgenetic alopecia in men and women. The effect is targeted at the hair root. In men, the 5% solution has been shown to be more effective than the 2% solution when used twice daily [8]. In women, the 2% and 5% solutions achieved approximately similar efficacy [9,10]. The enzyme sulfotransferase, which converts minoxidil into its biologically active form minoxidil sulfate, appears to play an important role. Several studies have shown that successful minoxidil application correlates with the amount of sulfotransferase in the hair follicles [11]. This is one possible explanation for why not all people with AGA respond to minoxidil. Goren et al. reported that after 16 weeks of continuous topical application of minoxidil, around 40% of patients showed a response to therapy [12]. It is believed that 60% of male patients do not respond to topical minoxidil therapy due to low baseline levels of sulfotransferase [14]. And analysis of sulfotransferase activity in plucked hair follicles appears to be an accurate predictor of women’s response to topical minoxidil treatment [11]. The first effects of minoxidil on hair growth usually set in after 6-8 weeks and reach their maximum after 12-16 weeks [13]. Minoxidil products (solution or foam) should be applied twice a day. If the application is stopped, the effects disappear again after a while. Occasional side effects of minoxidil can include itching, redness, inflammation and dandruff of the scalp [16]. Hypertrichosis can also occur, especially in women [10]. Another side effect is so-called “shedding”, which causes a temporary increase in hair loss at the start of treatment. If the patient is not informed, this may result in premature discontinuation of treatment [10,15].
| In men, AGA manifests as gradually increasing hair loss in the frontoparietal (“receding hairline”), frontal and/or vertex area. Around half of men over the age of 65 have AGA. Women are characterized by uniform hair thinning in the crown region, with the hairline at the frontal hairline remaining intact. AGA can occur in women after puberty, the prevalence in 70-year-olds is around 38%. |
| to [27] |
When oral finasteride fails: dutasteride as a possible alternative
Finasteride at a dosage of 1 mg/day has been shown to reduce the progression of AGA [17]. In a systematic review by Mella et al. oral finasteride was found to significantly improve hair growth and average hair count compared to placebo in ten studies [18]. In a 10-year follow-up study by Rossi et al. it was also found that daily use of finasteride in men with AGA over 5 years resulted in a sustained improvement in hair count in 21% of patients. The best hair growth results were achieved in the crown area of the scalp, while only minimal clinical improvements were seen in the forehead area [19]. The most common side effects of finasteride include sexual dysfunction; depending on the study, the frequency ranges from 1% to 40% [20,21]. The evidence base regarding the safety of long-term use of oral finasteride is rather limited.
Patients for whom oral finasteride is not effective may benefit from treatment with dutasteride. A study by Jung et al. towards. Male patients (n=31) received oral dutasteride 0.5 mg/d instead after a failed finasteride treatment attempt [22]. Approximately 80% showed clinical improvement after 6 months. Hair density increased by a statistically significant 10% and hair thickness increased by 20%. Similar side effects have been reported with dutasteride, such as reduced libido, impotence, ejaculation disorders and gynecomastia.
| Age-correlated changes in androgen metabolism The main cause of AGA is thought to be age-correlated changes in androgen metabolism, which lead to a progressive shortening of the anagen phase of hair growth and a decrease in hair follicle size [2,4]. In addition, there is a prolonged telogen phase, which leads to the regression of the hair follicles [5]. Due to the increased activation of androgen receptors in AGA, the hair follicles become increasingly shorter until they can no longer penetrate the epidermis [6]. Patients with AGA have been found to have elevated levels of dihydrotestosterone (DHT), and the scalp regions affected by AGA have increased androgen receptors [6]. Typically, AGA results in the loss of terminal hairs and replacement by small vellus hairs, usually in the temporal, parietal and mid-frontal areas of the scalp [7]. |
Low-level laser therapy (LLLT)
In LLLT, hair growth is stimulated by laser light emitted from laser diodes. LLLT can be performed at home, wearing a helmet with an integrated laser. In general, LLLT is well tolerated and the side effects are usually minor (e.g. dry scalp, itching, sensitivity and a feeling of warmth) [3]. A total of three studies on LLLT met the inclusion criteria of the S3 guideline. LLLT led to an increased hair count after 16 and 26 weeks of application under different protocols and with two different devices. However, no long-term follow-up was carried out [3]. A recent meta-analysis by Liu et al. found that 11 randomized, double-blind, controlled trials found a statistically significant improvement in hair density in patients treated with LLLT compared to placebo devices [23].
Last, but not least: PRP
Platelet-rich plasma (PRP) is obtained from the patient’s own blood. Three PRP studies were included in the S3 guideline [3,24–26]. Male and female patients were included in two of them [24,25]. An increase in hair density was observed with PRP treatment [24–26]. However, PRP was carried out in small samples, with different protocols and without a control group. According to Kaiser et al. PRP is particularly interesting as a treatment option for patients in whom several first-line treatment modalities have failed [2]. Regardless of the treatment option, it is important to discuss their expectations and goals with patients and to address the risks of side effects and treatment costs.
Literature:
- Deutsches Endokrinologisches Versorgungszentrum: Anlagebedingter Haarausfall, www.endokrinologen.de, (last access 19.01.2024)
- Kaiser M, et al.: Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clin Cosmet Investig Dermatol 2023; 16: 1387–1406.
- Kanti V, et al.: Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men – short version. JEADV 2018; 32(1): 11–22.
- Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol 2002; 37(8–9): 981–990.
- Kaliyadan F, Nambiar A, Vijayaraghavan S: Androgenetic alopecia: an update. Indian J Dermatol Venereol Leprol 2013; 79(5): 613.
- Ho CH, Sood T, Zito PM:Androgenetic alopecia. In: StatPearls. StatPearls Publishing; 2021.
- Rathnayake D, Sinclair R: Male androgenetic alopecia. Expert Opin Pharmacother 2010;11(8): 1295–1304.
- Olsen EA, et al.: A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. JAAD 2002; 47(3): 377–385.
- Lucky AW, et al.: A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. JAAD 2004; 50(4): 541–553.
- Blumeyer A, et al.: Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. JDDG 2011; 9: S1–S57.
- Roberts J, et al.: Sulfotransferase activity in plucked hair follicles predicts response to topical minoxidil in the treatment of female androgenetic alopecia. Dermatol Ther 2014; 27(4): 252–254.
- Goren A, et al.: Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol Ther 2015; 28(1): 13–16.
- Messenger A, Rundegren J: Minoxidil: mechanisms of action on hair growth. BJD 004; 150(2): 186–194.
- Goren A, et al.: Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther 2013; 27(3): 171–173.
- Randolph M, Tosti A: Oral minoxidil treatment for hair loss: a review of efficacy and safety. JAAD 2021; 84(3): 737–746.
- Suchonwanit P, Thammarucha S, Leerunyakul K: Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther 2019; 13: 2777.
- Kaufman KD, et al.: Finasteride in the treatment of men with androgenetic alopecia. JAAD 1998; 39(4): 578–589.
- Mella JM, et al.: Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol 2010; 146(10): 1141–1150.
- Rossi A, et al.: Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther 2011; 24(4): 455–461.
- Hirshburg JM, et al.: Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. J Clin Aesthet Dermatol 2016; 9(7): 56.
- Ganzer CA, Jacobs AR, Iqbal F: Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am J Men Health 2015; 9(3): 222–228.
- Jung JY, et al.: Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride. Int J Dermatol 2014; 53(11): 1351–1357.
- Liu K-H, et al.: Comparative effectiveness of low-level laser therapy for adult androgenic alopecia: a system review and meta-analysis of randomized controlled trials. Lasers Med Sci 2019; 34(6): 1063–1069.
- Jha AK, et al.: Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil. J Cosmet Dermatol 2019; 18(5): 1330–1335.
- Aggarwal K, et al.: Dermoscopic assessment of microneedling alone versus microneedling with platelet-rich plasma in cases of male pattern alopecia: a split-head comparative study. Int J Trichology 2020; 12(4): 156.
- Sinclair RD: Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol 2018; 57(1): 104–109.
- Beise U: Guideline Haarausfall, www.medix.ch/wissen/guidelines, (last access 22.01.2024).
DERMATOLOGIE PRAXIS 2024; 34(1): 48–49