Asthma and allergies are becoming increasingly important. Their treatment is often unsatisfactory, for example, many patients with allergic rhinitis complain of inadequate control of their nasal and ocular symptoms. A reformulated nasal spray containing azelastine and fluticasone may provide relief in the future.
The EAACI (European Academy of Allergy and Clinical Immunology) and WAO (World Allergy Organization), in one of their official communications to the congress, speak of the fact that more and more people worldwide are suffering from allergies or asthma. There is still a need for improvement in the treatment of these diseases. “For example, in an American survey, nearly half of patients with allergic rhinitis found that their symptoms were not optimally controlled,” explained Eli Meltzer, MD, USA [1]. “Symptoms around the nose and eyes in particular are poorly controlled by current monotherapy or combination therapies,” Dr. Meltzer emphasized. The newly formulated nasal spray with the active ingredients azelastine and fluticasone presented at the congress could possibly fulfill his wish for more effective treatment options.
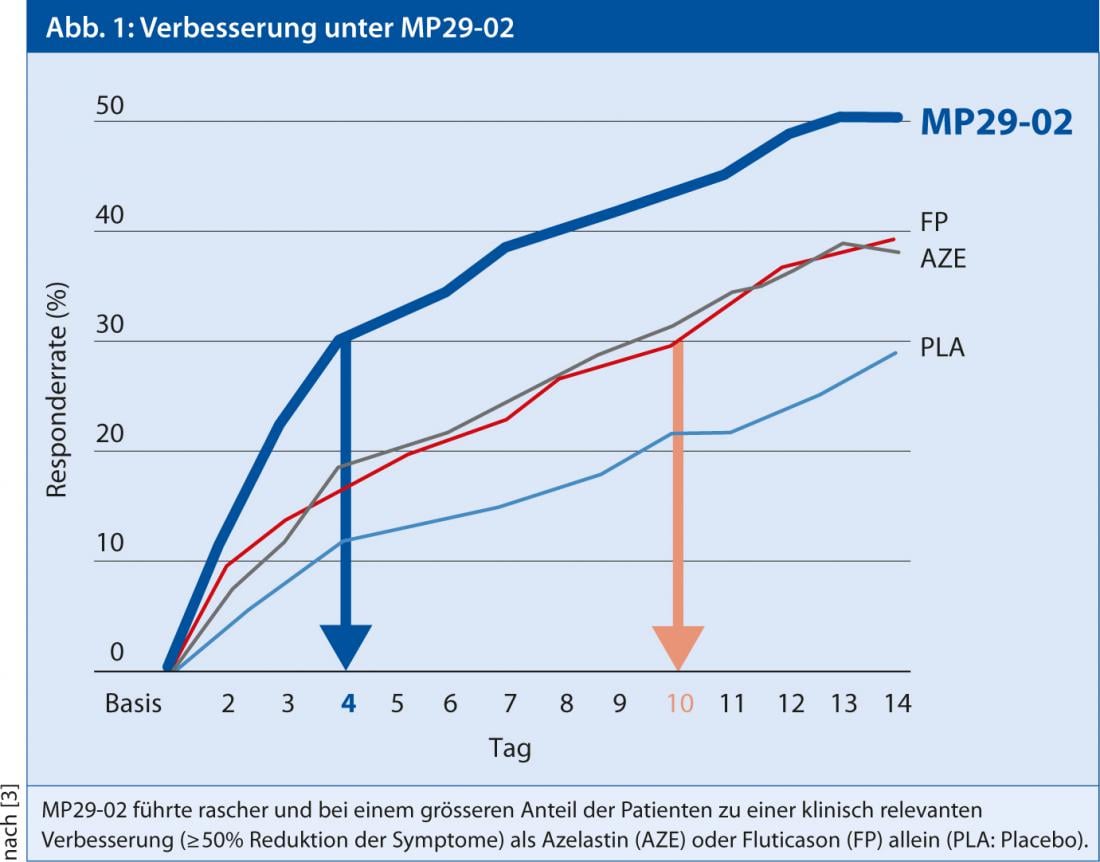
Better symptom control with new nasal spray
In a randomized, double-blind, placebo-controlled trial, patients with moderate to severe seasonal allergic rhinitis were treated with either MP29-02, a nasal spray containing fluticasone propionate, a spray containing azelastine, or placebo for 14 days after a seven-day placebo period [2]. At baseline, patients had an rTNSS (“Reflective Total Nasal Symptom Score”) of 18-19 (max = 24) and an rTOSS (“Reflective Total Ocular Symptom Score”) of 11-12 (max = 18). As was shown, all symptoms making up the rTNSS score (rhinorrhea, nasal congestion, itching, sneezing) improved significantly more under MP29-02 than when using a currently available product with the respective single component. This could also be demonstrated in a comparable manner for the rTOSS. “Relevant improvement in nasal symptoms was achieved days earlier with MP29-02 than with azelastine or fluticasone alone,” Dr. Meltzer concluded [3].
Source: EAACI-WAO Congress, June 22-26, 2013, Milan.
Literature:
- Meltzer EO, et al: Allergic rhinitis substantially impacts patient quality of life: findings from the Nasal Allergy Survey Assessing Limitations. J Fam Pract 2012; 61(2): 5-10.
- Hampel FC, et al: Double-blind, placebo-controlled study of azelastine and fluticasone in a single nasal spray delivery device. Ann Allergy Asthma Immunol 2010; 105: 168-73.
- Bachert C, et al: MP29-02 and Time to Response in the Treatment of Seasonal Allergic Rhinitis Compared to Marketed Antihistamine and Corticosteroid Nasal Sprays. European Academy of Allergy and Clinical Immunology (EAACI) Congress 2011, Abstract 285.