Germ cell tumors are rare overall, but at the same time are the most common tumors in young men. Management differs depending on the stage and prognosis group. Adequate, stage-appropriate therapy of metastatic germ cell tumors is a challenge in this regard.
Germ cell tumor is a rare entity overall, but at the same time the most common tumor in young men. Adequate, stage-appropriate therapy of metastatic germ cell tumors is a challenge in this regard. In the following, first-line therapy of primary metastatic tumors as well as therapy in recurrence will be discussed in more detail.
Introduction
Germ cell tumors account for approximately 2% of all malignancies and represent the most common neoplasm in males aged 15-45 years. In Switzerland, about 400 men contract the disease each year, and about 14 die each year. Thanks to the application of stage-specific therapy concepts, a cure rate of more than 90% can be achieved across all stages. Histologically, seminomas are distinguished from non-seminomatous germ cell tumors. While 95% of testicular tumors in males occur in the testis, approximately 5% are primarily located extragonadally. Known risk factors include cryptorchidism, history of testicular cancer, positive family history, infertility, and Klinefelter syndrome.
The most common symptom is a non-painful enlargement or swelling of the testicle. In rare cases, patients also notice signs of more advanced disease, such as back pain, dyspnea, weight loss, or neurologic symptoms. Diagnostically, clinical examination with palpation of the testes, sonography of both testes, and supplementary laboratory determination of the tumor markers HCG, AFP, and LDH are obligatory. The tumor markers can often already prove the neoplasia and are also used for therapy monitoring as well as follow-up control. Computer tomography of the thorax, abdomen and pelvis is always performed as well. Imaging of the head and bones is mandatory only in cases of extensive metastasis, especially pulmonary metastasis, very high tumor markers, evidence of clinical symptoms, or recurrence. If the patient wishes to have a child, sperm analysis and subsequent cryopreservation should always be performed before starting therapy.
Orchiectomy is often the first and also already curative therapeutic step in localized stage I. In germ cell tumors with high tumor burden, very high tumor marker constellation, or markedly symptomatic metastasis, however, orchiectomy is performed only after completion of systemic therapy.
The correct classification of tumor stages is relevant for the selection of therapy and the assessment of prognosis and optimizes the chances of cure. In this context, the use of chemotherapy, surgery and radiotherapy, the choice of drugs required, and the type and duration of their use are precisely defined by international consensus recommendations and guidelines.
In gonadal germ cell tumors, stage I disease is confined to the testis. Often in this case, orchiectomy is followed by surveillance alone (“Active Surveillanvce”) for seminomas and non-seminomas. However, in the presence of certain risk factors, adjuvant chemotherapy or radiation therapy may also be necessary in the localized stage, and rarely surgical resection of the retroperitoneal lymph nodes (RPLND).
Stage II and above is referred to as metastatic disease. All patients with a tumor stage >IIB and III require primary chemotherapy and are additionally assigned to a specific risk group according to the so-called IGCCCG risk classification ( International Germ Cell Cancer Collaborative Group) (Table 1) . Following systemic therapy, non-seminomas are treated with a residual tumor of >1 cm, a so-called residual tumor resection (RTR) is obligatory.
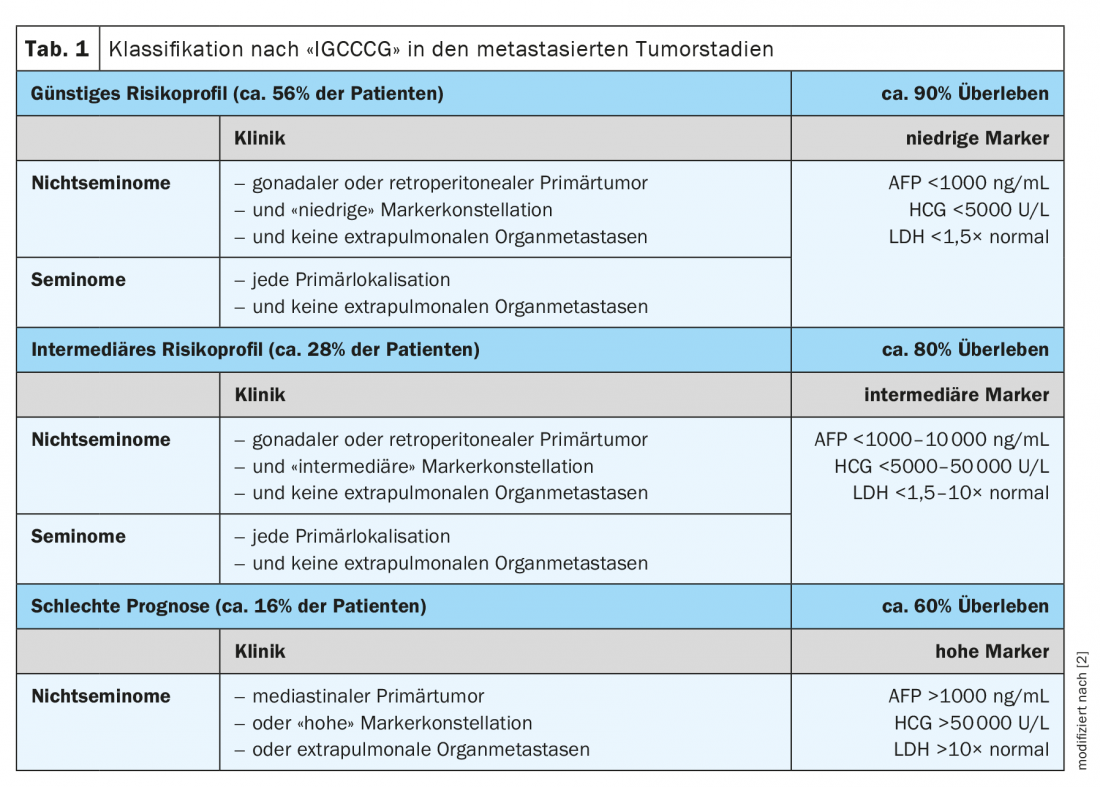
About 5-10% of all patients and 30% of primary already metastasized patients suffer a recurrence in the course. In this clinical context, as in the primary setting, a risk-adapted approach based on prognostic factors is important.
Neglect of treatment standards can lead to higher rates of treatment failure with the need for follow-up therapies or even death in both primary and relapse therapy. Therefore, patients, especially in the metastatic and relapsed situation or in the presence of rare disease scenarios (CNS involvement, late relapses, etc.), should be presented to clinics with high expertise for consultation.
First-line therapy in stage II
In rare stage IIA seminoma, outside of clinical trials, involved field radiotherapy remains the first treatment option. All seminoma stages from stage IIB are primarily treated with three cycles of combination chemotherapy with the drugs cisplatin, etoposide, and -leomycin (PEB) every 21 days or, alternatively, with four cycles of cisplatin/etoposide (PE) (Table 2) . Radiotherapy may also be discussed [1,2].

Currently, patients in Switzerland can also be enrolled in a Phase III clinical trial (SAKK 01/18). Patients here receive combined chemo-radiotherapy (1 cycle of carbo-platin AUC 7 followed by involved node irradiation with 24 Gy in II A or 1 cycle of PE followed by involved node radiotherapy with 30 Gy in II B). Further Phase II trials in Germany or
in the USA are currently investigating surgery alone without adjuvant therapy (PRIMETEST, NCT 02797626).
Patients with normal tumor marker constellation and image morphologically suspicious retroperitoneal lymph nodes (usually 1-2 cm in diameter) represent a special subpopulation. In these patients, short-term imaging follow-up 6-8 weeks later with close concurrent marker monitoring is recommended. RPLND may be sought in patients with nonseminomas whose lymph nodes (LK) are unchanged with tumor markers remaining normal to rule out teratoma. Alternatively, further control is performed. All patients with tumor markers that increase during the course and/or rapidly size-progressing LK in imaging control require prompt initiation of chemotherapy according to the risk stratification for advanced tumors [3].
Treatment of all metastatic seminoma from stage IIC consists of chemotherapy according to the PEB regimen (cisplatin, etoposide, bleomycin) [1,2]. The duration and intensity are based on the IGCCCG risk classification. If the prognosis group is good, patients are treated with a total of three cycles of PEB at 21-day intervals. The intermediate or poor prognosis group is treated with four cycles at the same interval. If there is a contraindication to bleomycin, four cycles of PE (cisplatin, etoposide) are equivalent to three cycles of PEB in a good prognosis group. In the intermediate and poor prognosis group, four cycles of PEI (cisplatin, etoposide, ifosfamide) are applied instead of four cycles of PEB.
Stage II non-seminomas with elevated tumor markers are treated according to their IGCCCG staging according to the therapy algorithm for advanced tumor stages [2].
First-line therapy in stage III
Standard therapy is according to risk stratification according to the IGCCCG risk classification regardless of histology. It consists of chemotherapy with three (for good prognosis group) or four cycles (for intermediate or poor prognosis group) of PEB at 21-day intervals (alternatively, 4 cycles of PE for good prognosis or 3 – 4 cycles of PEI for intermediate/poor prognosis) [2].
In recent years, intensified chemotherapy strategies have been investigated especially for the intermediate and poor prognosis groups. In this regard, for the first time, a prospective randomized study (GETUG13) by a French research group demonstrated an advantage in progression-free survival by subsequent therapy intensification in patients with inadequate marker decline in the first cycle [4].
American data for the TIP regimen with paclitaxel, ifosfamide, and cisplatin, previously used only in salvage therapy, also showed very good long-term results in this patient population in a phase II study, but without significant advantage compared with standard therapy [5]. The addition of paclitaxel to PEB in the intermediate-risk group of patients in an EORTC trial showed a 12% significant improvement in progression-free survival, but without significant prolongation of overall survival concomitant with increased toxicity [6].
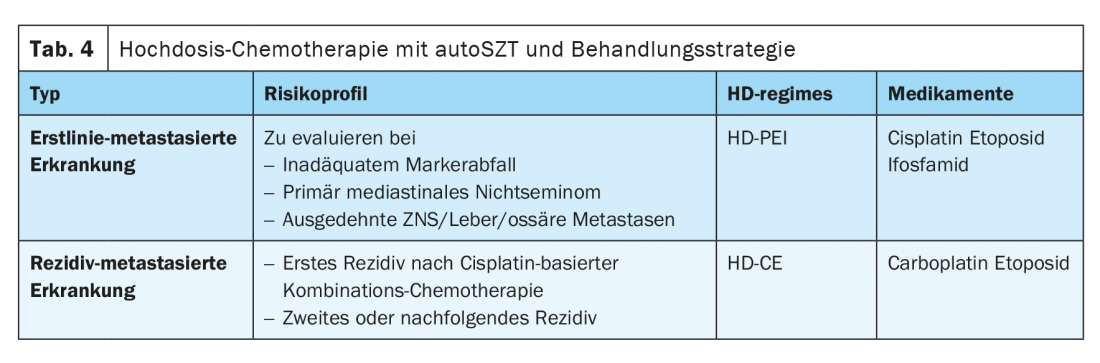
In many cases, the value of primary high-dose chemotherapy (HDCT) with autologous stem cell support in primary therapy has also been explored, especially in the group of patients with poor prognostic features according to IGCCCG. In a multicenter phase II study of the German Testicular Tumor Study Group, sequential HDCT with cisplatin, etoposide, and ifosfamide (HD-PEI) was investigated and demonstrated a long-term survival rate of 75%. A randomized phase III trial from the United States comparing the administration of four cycles of PEB versus two cycles of PEB followed by two cycles of high-dose chemotherapy with carboplatin, etoposide, and cyclophosphamide (CEC) failed to show a general advantage in favor of HDCT. Only patients whose tumor markers had not decreased adequately over time benefited more from treatment in the high-dose arm. The published randomized phase III EORTC trial comparing four cycles of PEB with sequential high-dose PEI administration also showed no statistically significant benefit in favor of HDCT [7,8]. Thus, the use of HDCT in primary therapy in patients with unfavorable prognostic factors remains not a standard of care at present.
Primary HDCT may still be useful in individual cases, particularly in patients with inadequate marker decline after the first conventionally dosed cycle of therapy, in patients with primary mediastinal nonseminoma, and in patients with CNS metastases or extensive liver or bone metastasis. This approach should be decided only in consultation with experienced centers, and the data should be recorded in a registry if possible.
Residual tumor resection (RTR) after first-line therapy.
In patients with seminoma and post-chemotherapy tumor residuals, RTR is not mandatory. For residuals >3 cm, PET-CT can be discussed at the earliest eight weeks after completion of chemotherapy. Only in this constellation does PET constitute an indication worth considering at all. In PET-positive patients, the examination must first be repeated in the interval by means of CT follow-up or, if necessary, a biopsy must be evaluated in order to reliably exclude possible false-positive findings with a simultaneously normal constellation of markers [9].
In all non-seminoma patients with tumor residues >1 cm, RTR should be performed early, approximately four to a maximum of eight weeks after completion of chemotherapy with the goal of complete removal of all tumor residues. This often complex procedure should only take place at an interdisciplinary center with appropriate expertise. If vital tumor cells are detected in the resectate, the further procedure is not clearly defined. Both repeat therapy with two cycles of chemotherapy and follow-up alone can be discussed if the tumor cell percentage in the resectate is vital and >10% [10].
Salvage therapy
Treatment of patients with seminoma or non-seminoma and recurrence from a stage I is analogous to the treatment algorithms for patients with primary metastatic disease [1].
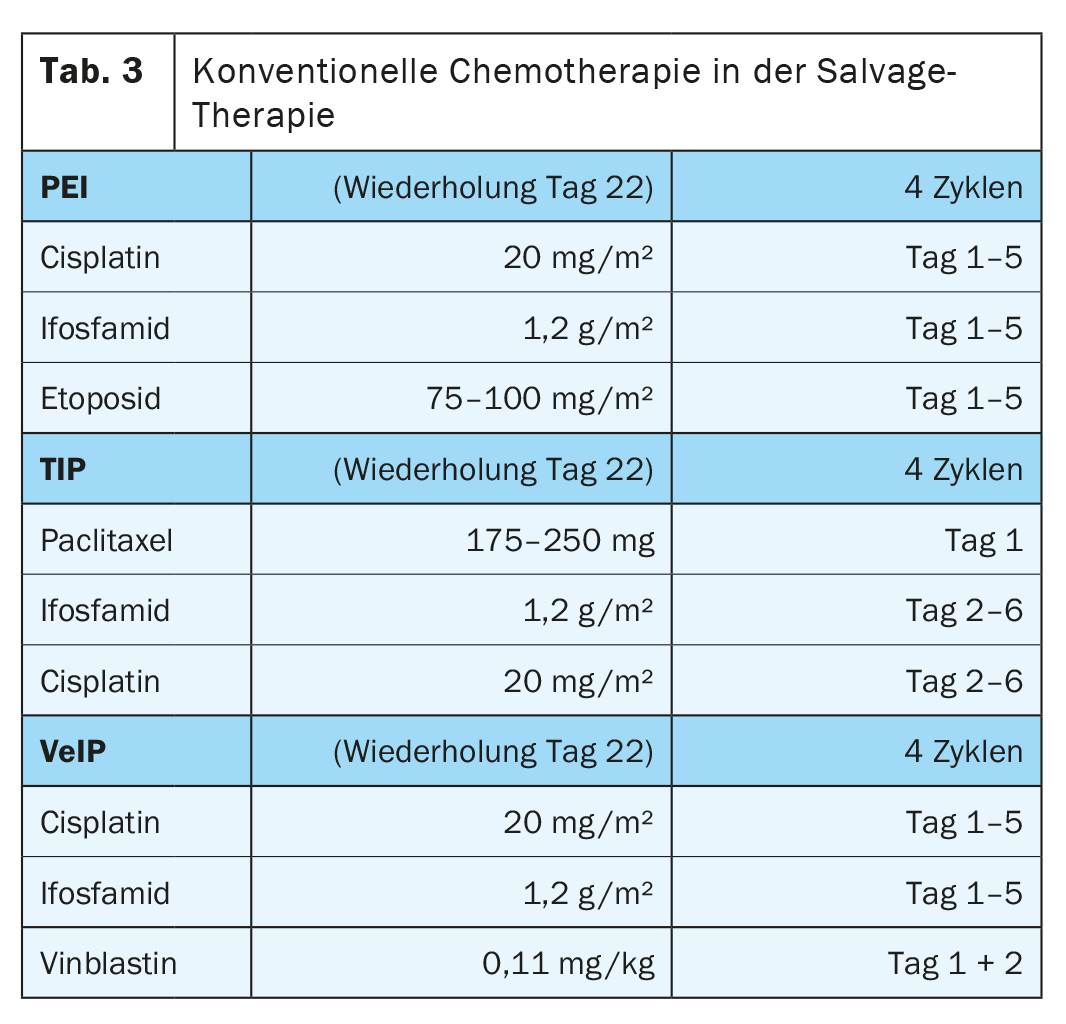
Patients who develop a recurrence after primary chemotherapy with at least three cycles of cisplatin-containing therapy receive a repeat intensive chemotherapeutic regimen. This is supplemented by subsequent RTR in non-seminoma patients. Treatment options in principle include conventional cisplatin-based salvage therapy (CDCT) or sequential high-dose chemotherapy with autologous stem cell transplantation (HDCT). In certain scenarios, salvage surgery alone is also indicated (e.g., Growing Teratoma) [11].
The treatment that is considered for the individual patient depends primarily on the time of occurrence of the disease relapse and on certain risk factors. Depending on the risk factors, a distinction is made between five prognosis categories. In this context, the estimated 2-year Kaplan-Meier PFS is 75% for patients in the very low risk group, 51% in the low risk group, 40% in the intermediate risk group, 26% in the high risk group, and 6% in the very high risk group [12].
Conventional cisplatin-containing salvage therapy.
The regimens combine cisplatin (which cannot be replaced by carboplatin) and ifosfamide with either etoposide (PEI), with vinblastine (VeIP), or with paclitaxel (TIP) without clear superiority of any particular therapeutic combination (Table 3) . The standard of combination chemotherapy is the administration of four cycles each 21 days apart [3].
Sequential high-dose chemotherapy with autologous stem cell transfusion (HDCT).
The combination of carboplatin and etoposide (CE) forms the basic framework of HDCT. Today, this is performed at almost all centers worldwide in the form of a sequential therapy with two to three high-dose cycles of CE (Tab. 4). Improved supportive therapy and the use of autologous peripheral blood stem cells (PBSC) significantly shortened -hematopoietic reconstitution times and thus reduced the initially high treatment-related lethality from more than 10% to less than 3%.
The value of HDCT as the first salvage therapy is still controversial and the subject of current discussions. Subgroup analysis of a retrospective study of nearly 1600 records of patients with primary salvage therapy demonstrated an advantage in favor of HDCT compared with CDCT in the first recurrence. These results are contrary to data from a prospective randomized trial that demonstrated no clear benefit for HDCT in the first recurrence [13,14].
A global randomized phase III trial comparing the conventional regimen with TIP and sequential high-dose chemotherapy (CE) (TIGER trial) is currently prospectively validating the benefit of HDCT in the first relapse. Patients can also be included in Switzerland.
In the second or subsequent relapse, HDCT can also still achieve long-term remission in a small proportion of patients. However, the small size and heterogeneity of the patient populations studied also complicates the interpretation of available study results [15].
Residual tumor resection after salvage therapy
The overall proportion of patients with vital, undifferentiated histology is higher after relapse chemotherapy. Therefore, after completion of systemic therapy, RTR should be evaluated in all patients with nonseminomas and detectable residuals. In individual patients without marker normalization or multiple, chemotherapy-refractory recurrences, resection in the sense of a so-called “desperation surgery” can still lead to remission in exceptional cases, especially in the presence of singular and well resectable tumor manifestations and sole AFP elevation [3,16].
Late relapses
Recurrences that occur at least two years after the last cisplatin-containing chemotherapy are referred to as late recurrences. In resectable disease, primary surgical excision is the treatment of choice. In cases of non-resectable findings and/or very high tumor markers (especially HCG), systemic therapy should be given first (either in the form of CDCT or HDCT) and resection should be performed after completion of chemotherapy. More frequently, late recurrences in the resectate show unfavorable histologies with transformation into sarcomas or adenocarcinomas, among others [16].
CNS Infestation
CNS metastases rarely occur. These may occur either synchronously at primary diagnosis or at recurrence. Isolated cerebral recurrence is found in only about 2% of patients. In addition to identifying prognostic factors, a retrospective study compared the individual treatment modalities of chemotherapy, radiotherapy, and resection and examined patient survival at both primary diagnosis and recurrence. Thus, it could be shown that, after diagnosis, radiatio and/or resection do not necessarily have to be performed in addition to chemotherapy. In contrast, in the relapse setting, maximal utilization of all forms of therapy, including HDCT, appears to significantly improve patient survival [17].
Recurrence after HDCT and autoSZT
Patients with multiple recurrences or patients with recurrences after high-dose chemotherapy are rarely cured. However, through the well-coordinated use of palliative chemotherapy, palliative tumor resection if necessary, or even palliative radiation, symptom relief and thus a better quality of life can often be achieved.
In addition to paclitaxel, the substances oxaliplatin and gemcitabine also show efficacy and are used either as single substances or in various combinations. The GOP regimen, which combines oxaliplatin with gemcitabine and paclitaxel, has been particularly successful and is able to induce long-term remissions in individual patients even in relapse after prior HDCT. Palliative efficacy has also been demonstrated for the use of oral etoposide. Unfortunately, there is no effective alternative besides chemotherapy so far, especially tyrosine kinase and checkpoint inhibitors did not show efficacy in clinical trials [2,3,18].
Take-Home Messages
- Germ cell tumor is the most common tumor in young men.
- In the metastatic stage, classification is into three prognostic groups: good, intermediate, and poor, depending on marker level and visceral involvement.
- Chemotherapy with PEB is the standard therapy for advanced stage tumors; alternatively, PE or PEI may be used.
- The number of cycles in primary therapy is based on the prognosis group.
- In non-seminomas, residual tumor resection is obligatory for residuals >1 cm.
- In the salvage setting, either conventional or high-dose chemotherapy with autologous stem cell support is performed.
- Patients with CNS and late relapses represent a special type.
Literature:
- Honecker F, et al: ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018; 29(8): 1658-1686.
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997; 15: 594-603.
- Onkopedia Guidelines Germ cell tumors of the male. www.onkopedia.com (last accessed 03/2021)
- Fizazi K, et al: Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol. 2014; 15: 1442-1450.
- Feldman DR, et al: Paclitaxel, Ifosfamide, and Cisplatin Efficacy for First-Line Treatment of Patients with Intermediate- or Poor-Risk Germ Cell Tumors. J Clin Oncol. 2016; 34(21): 2478-2483.
- de Wit R, et al: Randomized phase III study comparing paclitaxel-bleomycin, etoposide, and cisplatin (BEP) to standard BEP in intermediate-prognosis germ-cell cancer: intergroup study EORTC 30983. J Clin Oncol. 2012; 30(8): 792-799.
- Motzer RJ, et al: Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol. 2007; 25: 247-256.
- Daugaard G, et al: A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974). Ann Oncol. 2011; 22: 1054-1061.
- Cathomas R, et al: Questioning the Value of Fluorodeoxyglucose Positron Emission Tomography for Residual Lesions After Chemotherapy for Metastatic Seminoma: Results of an International Global Germ Cell Cancer Group Registry. J Clin Oncol. 2018. DOI: 10.1200/JCO.18.00210.
- Heidenreich A: Residual tumor resection following inductive chemotherapy in advanced testicular cancer. Eur Urol. 2007; 51: 299-301.
- Lorch A, et al: Sequential versus single high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: long-term results of a prospective randomized trial. J Clin Oncol. 2012; 30: 800-805.
- Lorch A, et al: Prognostic Factors in Patients With Metastatic Germ Cell Tumors Who Experienced Treatment Failure With Cisplatin-Based First-Line Chemotherapy. J Clin Oncol. 2010; 28: 4906-4911.
- Lorch A, et al: Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol. 2011; 29: 2178-2184.
- Pico JL, et al: A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol. 2005; 16: 1152-1159.
- Adra N, et al: High-Dose Chemotherapy and Autologous Peripheral-Blood Stem-Cell Transplantation for Relapsed Metastatic Germ Cell Tumors: The Indiana University Experience. J Clin Oncol. 2017; 35(10): 1096-1102.
- Albers P, et al: Guidelines on Testicular Cancer: 2015 Update. Eur Urol. 2015; 68(6): 1054-1068.
- Feldman DR, et al: Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options-An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol. 2016; 34: 345-351.
- Bokemeyer C, et al: Combination chemotherapy with gemcitabine, oxaliplatin, and paclitaxel in patients with cisplatin-refractory or multiply relapsed germ-cell tumors: a study of the German Testicular Cancer Study Group. Ann Oncol. 2008; 19: 448-453.
InFo ONCOLOGY & HEMATOLOGY 2022; 10(5): 6-10.











