In one in five patients with a chronic cough, the cause remains unexplained and the question of symptom-relieving treatment options arises. P2X3 antagonists inhibit the transmission of cough stimulus signals to the brain stem and the somatosensory cortex, where the cough reflex is regulated. Gefapixant is a representative of this group of active substances, which was approved last year based on clinical trial data showing a significant reduction in 24-hour cough frequency. Taste disturbances were reported as the most common side effect.
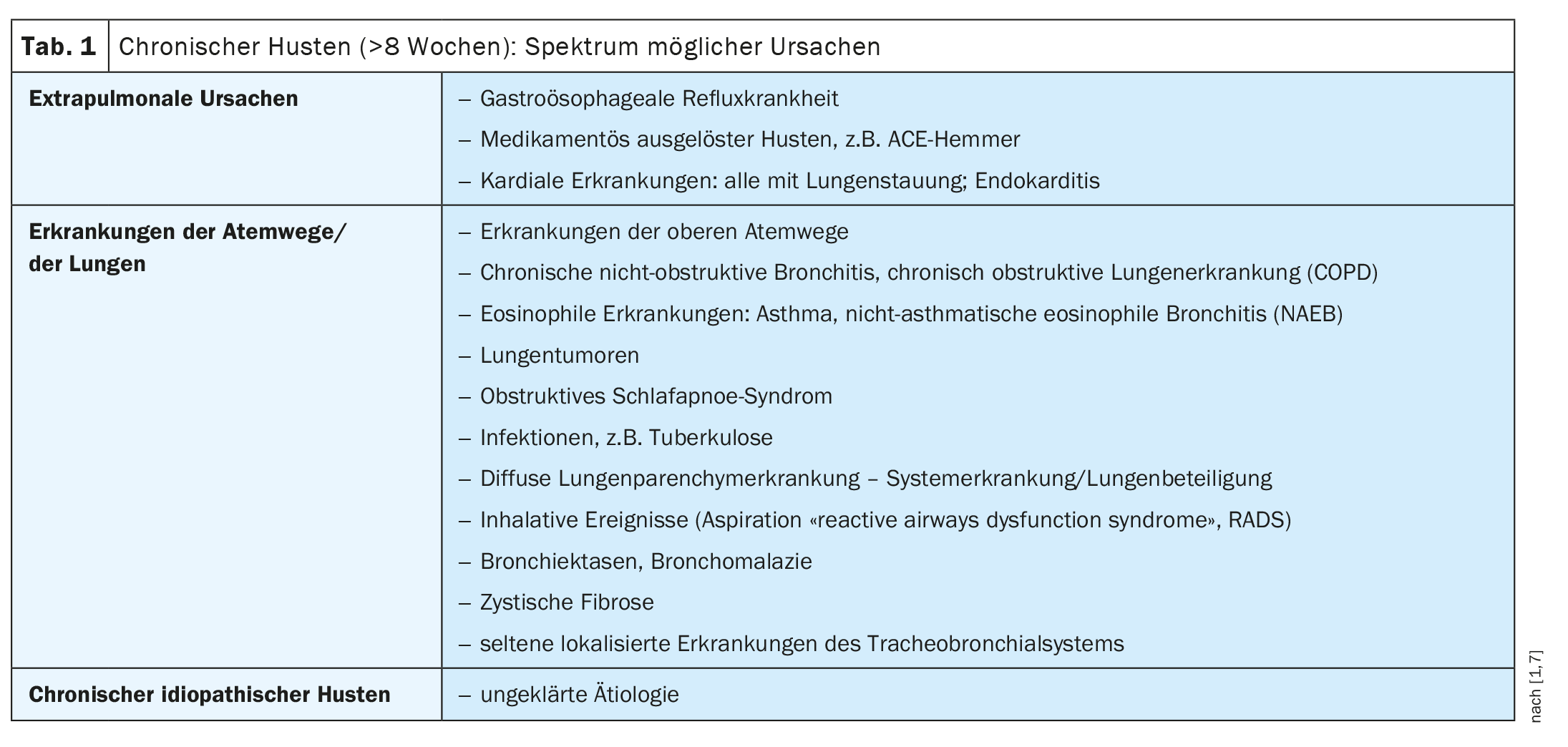
A cough is a very common reason for consultation in primary care [1,2]. Acute and subacute coughs are usually infectious and subside after a few days or weeks. A cough that lasts longer than eight weeks is described as chronic. Chronic cough is a possible symptom of a wide range of pneumological and non-pneumological diseases. In patients with chronic cough, a chest X-ray in two planes and a pulmonary function test should be initiated as a basic diagnostic procedure to ensure that no serious disease is overlooked [10].
If no specific cause can be identified: What next?
In around 20% of cases of chronic cough, none of the pulmonary or extrapulmonary causes listed in Table 1 are present, so that we speak of idiopathic chronic cough or refractory cough of unexplained etiology, according to Prof. Dr. Marek Lommatzsch from the Department of Pneumology, University Medicine Rostock [1,2]. An unexplained chronic cough can be stressful and restrict everyday life. Conventional non-opioid antitussives (e.g. thyme, ivy, ribwort) and opioid antitussives (e.g. morphine sulphate, dextrometorphan, noscaine, codeine/dihydrocodeine) are not officially approved for long-term therapy and usually do not lead to successful treatment [1]. The active ingredient gefapixant (Lyfnua®) has a different mechanism of action [3]. It is the first approved representative of the antagonists of P2X3 receptors, a new class of antitussives. Lyfnua® has been on the market in Switzerland since 2022.
P2X3 receptor antagonist: innovative therapeutic strategy
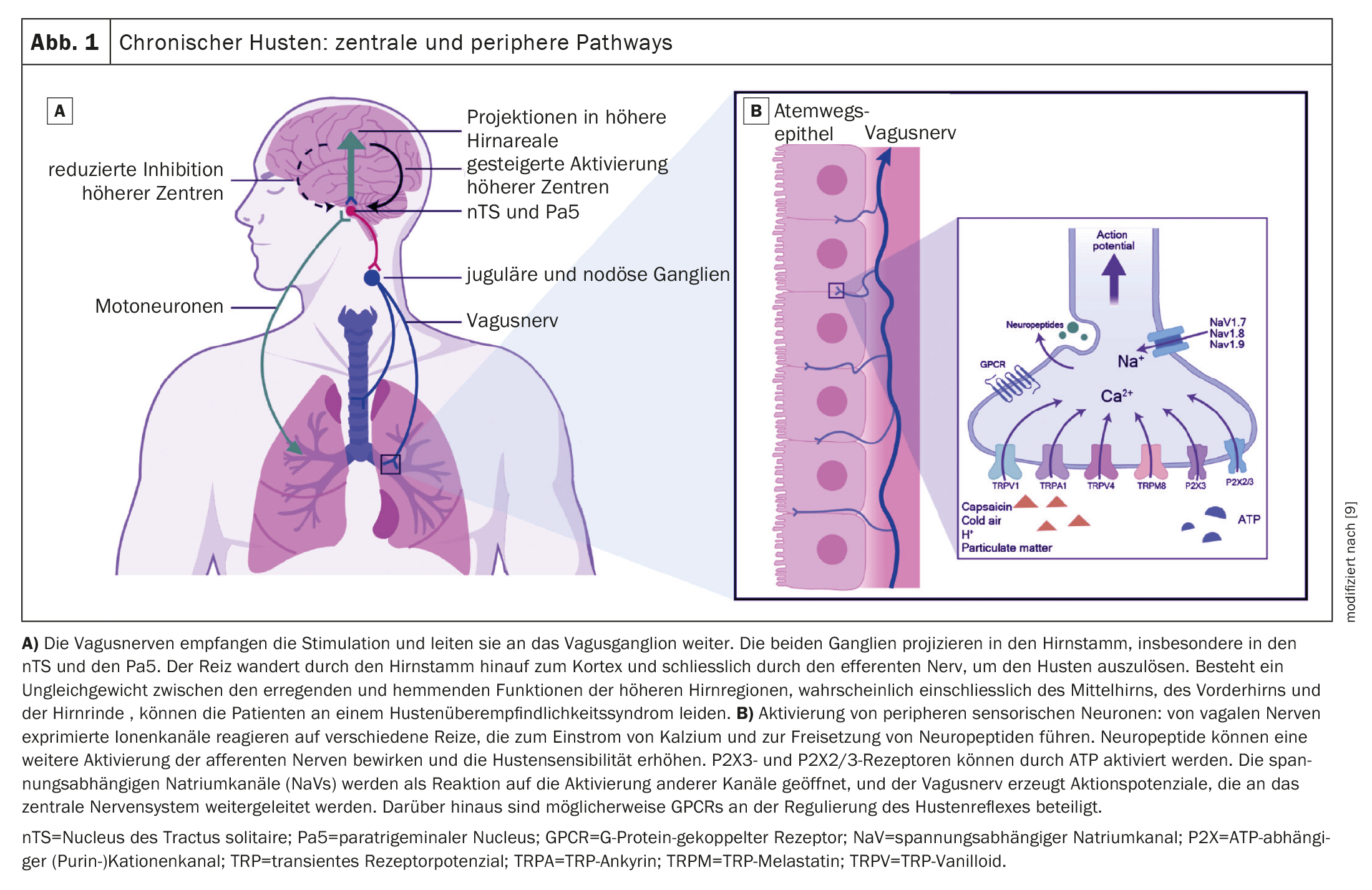
Hypersensitivity of the cough reflex, which includes increased peripheral and central sensitization of the cough reflex, is one of the main features of idiopathic chronic cough [4]. “The lungs are richly innervated,” says Prof. Lommatzsch. There are over 2000 sensory nerve endings per mm2 of epithelium and various receptors. Both dextromethorphan, a central cough suppressant whose effects are based, among other things, on antagonism at the NMDA receptor and agonism at the sigma-1 receptor, and narcodin, a peripheral cough suppressant that blocks cough sensors, afferent or efferent neurons, can reduce the frequency of coughing, but are only used to a limited extent due to their side effects [5].
The orally available P2X3 receptor antagonist gefapixant has emerged as an effective alternative treatment strategy for patients with idiopathic chronic cough. It is known that overexpression of the receptor P2X3 in the vagal afferent neurons of the airways can cause hypersensitization of sensory neurons associated with the pathogenesis of chronic cough [6]. Specifically, the mechanism of action of Gefapixant is that ATP-dependent ion channels on sensory neurons in the airways are blocked, which alleviates the cough stimulus. The latter can be explained by the fact that under inflammatory conditions, ATP is released by mucosal cells of the airways and the binding of extracellular ATP to P2X3 receptors is recognized by C-fibers as a damage signal and perceived as a cough stimulus.
COUGH-1 (n=730) and COUGH-2 (n=1314) were both double-blind, randomized, placebo-controlled pivotal trials in a parallel group design [8]. COUGH-1 was conducted at 156 sites in 17 countries and COUGH-2 at 175 sites in 20 countries. The studies included participants who were at least 18 years old and had been diagnosed with refractory chronic cough or unexplained chronic cough of at least one year’s duration. Both were three-arm studies; in COUGH-1, 243 subjects received placebo, 244 received gefapixant 15 mg (2× daily) and 243 received gefapixant 45 mg (2× daily) and in COUGH-2, 435 were randomized to the placebo group, while 440 subjects received gefapixant 15 mg (2× daily) and 439 received gefapixant 45 mg (2× daily). As a result, gefapixant 45 mg (2× daily) showed a significant reduction in 24-hour cough frequency at week 12 in COUGH-1 and at week 24 in COUGH-2 (p=0.041 and p=0.031 respectively) compared to placebo. The most common adverse events were related to taste disturbances. The speaker recommended informing patients in advance of treatment that Gefapixant is an effective antitussive, but that taste-related side effects may occur. In a pooled post hoc analysis published in the American Journal of Respiratory and Critical Care Medicine , several patient-reported outcomes (PROs) collected in COUGH-1 and COUGH-2 were evaluated [11]: Leicester Cough Questionnaire (LCQ), Visual Analog Scale of Cough Severity (VAS), Cough Severity Diary (CSD). The results show that a greater proportion of those who received gefapixant 45 mg achieved clinically meaningful improvements in these PROs compared to placebo. With regard to taste disturbances as the most common side effect, the authors point out that these were not serious and did not lead to hospitalizations.
Literature:
- “Pneumology”, Prof. Dr. med. Marek Lommatzsch, DGIM Internists Update Seminar, 10-11.11. 2023, Wiesbaden/Livestream
- Spiesshoefer J: Chronic cough: diagnosis and targeted therapeutic approach. SUPPLEMENT: Perspectives in Pneumology & Allergology. Dtsch Arztebl 2023; 120(40): [20]; DOI: 10.3238/PersPneumo.2023.10.06.03
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch,(last accessed 19.12.2023)
- Gibson PG, Vertigan AE: Management of chronic refractory cough. BMJ 2015; 351, h5590.
- Gibson P, et al: Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest 2016; 149: 27-44.
- Smith JA, et al: Gefapixant, a P2X3 receptor antagonist, for the treatment of ssractory or unexplained chronic cough: a randomized, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir. Med 2020; 8: 775-785.
- Kardos P: Chronic cough. Pneumology 2023; 77(08): 574-585.
- McGarvey LP, et al: Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomized, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022; 399, 909-923.
- Zhao K, et al: Similarities and differences between the cough suppression test and the cough challenge test. Ther Adv Respir Dis 2023 Jan-Dec; 17: 17534666231162246.
- Kardos P: Acute and chronic cough – is there anything new? [Acute and chronic cough-What is new?]. Pneumologe (Berl) 2020; 17(6): 433-442.
- Birring SS, et al: Efficacy and Safety of Gefapixant for Refractory or Unexplained Chronic Cough over 52 Weeks. Am J Respir Crit Care Med 2023; 207(11): 1539-1542.
HAUSARZT PRAXIS 2024; 19(1): 34-36 (published on 22.1.24, ahead of print)