Influenza is the most common vaccine-preventable infectious disease. Nevertheless, it consistently tops the list of infectious diseases in Europe – with the exception of the past two seasons, when the COVID 19 pandemic strongly influenced the picture. But how has the pandemic affected population-based immunity to influenza and what can be expected from it for post-pandemic seasons?
The infection itself is one problem with influenza. The other is the increase in risk for secondary diseases such as myocardial infarction (up to 10-fold increase) or apoplectic insult (up to 8-fold increase), recalled Prof. Dr. Susanne Herold, Medical Clinic V – Internal Medicine, Infectious Diseases and Hospital Hygiene, University Hospital Giessen and Marburg (D) [1]. During the recent strong 2017/2018 influenza season, two-thirds of cases were Yamagata lineage influenza B infections. The problem at the time was that the influenza vaccine did not include this line. Since then, therefore, the quadrivalent influenza vaccine (QIV) with 2 B and 2 A lines has always been recommended.
Regarding the recommendations, Prof. Herold pointed out that simultaneous vaccination against influenza and COVID-19 is possible and that no time interval is required, which should unquestionably improve compliance among patients. In addition, it is new that older persons (in Switzerland from the age of 65 years) can be vaccinated with a high-dose vaccine (box, tab. 1), which contains a higher amount of antigen.
That older adults suffer significantly more influenza-associated morbidity and mortality is well known. The expert cited data from Germany, according to which in the 2017/2018 season the proportion of hospitalizations with patients over 60 years of age was 58%, and in the case of deaths associated with influenza, the proportion from this age group even increased to 86%. The background to this is immune senescence, which leads to poorer efficacy in the elderly.
Better immune response in the elderly
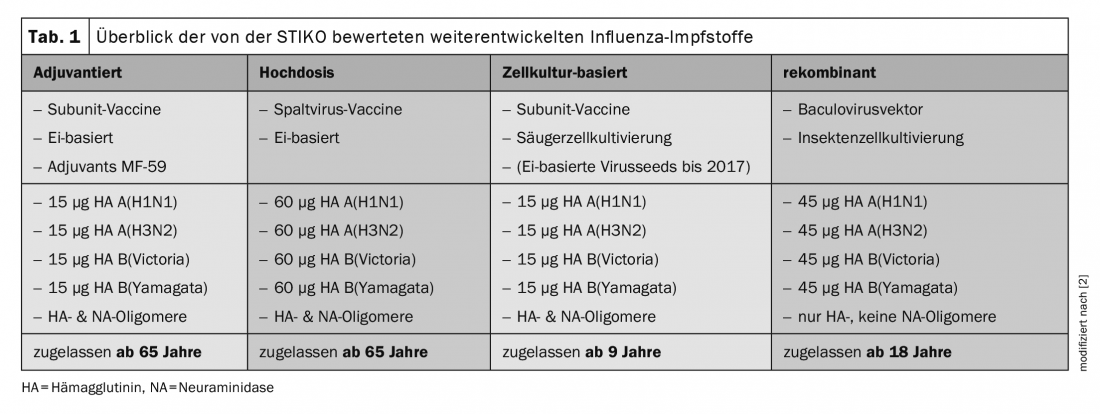
The Standing Commission on Vaccination (STIKO) in Germany has addressed the problem and reviewed new licensed vaccines in this context. Four different approaches have been studied: Influenza vaccines to adjuvant, taking higher doses (hemagglutinin increased 4-fold compared to standard vaccine), cell culture-based vaccines have been looked at, and recombinant ones have also been thought to elicit a better vaccine response than the egg-based split vaccine [2]. “However, the result was that only the high-dose vaccine resulted in a better immune response in elderly patients,” Prof. Herold summarized. Based on the results, the German scientists therefore recommend that all persons aged 60 years and older (65 years and older in Switzerland) receive annual vaccination against seasonal influenza in the fall with an inactivated quadrivalent influenza high-dose vaccine with the current antigen combination recommended by the WHO.
Among others, this was based on an American-Canadian multicenter study [3]. In their intention-to-treat (ITT) analysis, of 32,000 participants, 1.4% had influenza with the high-dose vaccine and 1.9% with the standard dose. “Now that doesn’t sound like such a terrible deal,” Prof. Herold said, “but the relative vaccine activity in the high-dose group was 24.2% higher, and immunogenicity was also significantly increased.”
|
Recommendations of the FOPH The Federal Office of Public Health (FOPH) recommends flu vaccination for people at increased risk of complications:
To better protect these people, not only they themselves, but also everyone in regular, close contact with them should be vaccinated against the flu. These include close family members, infant caregivers, and health professionals. The recommended time period for vaccination is from mid-October until the beginning of the flu season. Influenza vaccination can be given at the same time as, before, or after COVID-19 vaccination. No minimum interval between mRNA vaccination and other vaccinations is recommended [4]. |
New developments: mRNA-based vaccines
Since COVID-19, the advantages of an mRNA-based vaccine have been known. Depending on how the mRNA is modified, it can act like an adjuvant. Since mRNA is recognized by the host cell as a viral antigen, TLR or interferon signaling pathways can be triggered directly, thus enhancing the immune response. Phase 1/2 studies are currently ongoing to investigate safety and reactogenicity as well as immunogenicity in terms of antibody titers.
Another goal of vaccine development is the “universal influenza vaccine”. The reason for the annual vaccination is antigenic drift, i.e. mutations in the head of hemagglutinin (HA) and neuraminidase (NA). Therefore, the target structures of the approach are highly conserved epitopes such as the HA strain. “If you can achieve antibodies against the strain, you don’t have to keep adapting,” Prof. Herold explained. Similarly, the matrix protein M2e or even neuraminidase alone could be addressed, because it is known that this retains a relatively high cross-reactivity within a subtype and can, for example, also act against H5N1 if it also acts against H1N1.
Finally, the infectiologist presented new delivery methods by enhancing mucosal immunity through local deposition. “That’s what we’re already doing in children with the live attenuated vaccine and the nasal spray.” The goal is to induce resident memory T and B cells, especially in nasopharyngeal-associated lymphoid tissue and bronchus-associated lymphoid tissue, in the upper and, if possible, also in the lower respiratory tract, and to induce mucosal antibodies. “The mucosa is the first line of defense. If you combine this approach with other systemic vaccines, you may be able to achieve better efficacy.” Thus, the problem of low immunogenicity in terms of preventing infection and decreasing Ak titers after about 6 months could be addressed in the future.
62nd Congress of the German Society for Pneumology and Respiratory Medicine e.V. in Leipzig (D), May 25-28, 2022.
Literature:
- Herold S: Influenza. Symposium “Update – Vaccinations in pneumological infections” in the context of the DGP Congress, 26.05.2022.
- Michaelis K, et al: Epid Bull 2021; 1: 3-25.
- DiazGranados CA, et al: N Engl J Med 2014; 371: 635-645; doi: 10.1056/NEJMoa1315727.
- Federal Office of Public Health; www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/grippe.html#-2084790127 (last accessed Aug. 24, 2022).
InFo PNEUMOLOGY & ALLERGOLOGY 2022; 4(3): 34-36.