Since the discovery and introduction of the substance class of biologics, we have been able to achieve unprecedented therapeutic successes in the treatment of inflammatory dermatoses. The very specific action of these drugs also reduces adverse effects on other organs, which has been shown empirically in studies as well as after market introduction. Nevertheless, the specialist must be particularly prepared for some side effects in order to recognize and interpret them correctly. This article provides an overview of cutaneous reactions in biologics patients.
The protein-based biologics specifically intervene in biological signaling pathways and inhibit or enhance the action of messenger substances. Compared to the classical small molecules, the effects of biologics in the human organism are much better understood.
Injection and infusion reactions
By far the most common side effects of biologics are reactions that occur at or shortly after administration of the drug. With biologics injected subcutaneously, redness around the injection site is relatively common (up to 20%). In addition, pain may occur directly during the injection. These injection reactions disappear again after a few hours up to a maximum of two or three days and are classified as mild in terms of dangerousness. Control that these reactions occur only around the injection site; treatment, such as topical steroids or changing the preparation, is necessary only if the intensity of the reaction increases over time. Pain from injection is more frequent when the drug is stored in the refrigerator and injected directly with the temperature of 4 degrees. Therefore, we recommend storing the drug at room temperature for half an hour before injection – this measure can greatly reduce pain in many cases.
With biologics given i.v., systemic reactions also sometimes occur during or immediately after infusion. The spectrum of intensity of these reactions is very wide: we refer to the specific guidelines for the administration of the respective drugs, as these reactions, although rare (about 1%), must be answered with specific measures, such as slowing down the infusion rate.
Paradoxical psoriasis
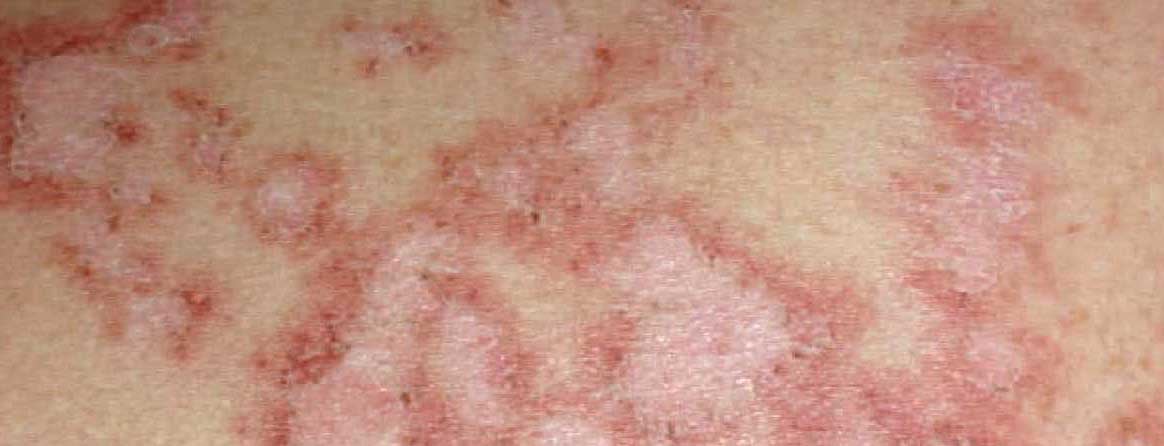
Tumor necrosis factor (TNF)-α antagonists have a specific, relatively common side effect: paradoxical psoriasis. This occurs in approximately 0.5% of all patients treated with a TNF antagonist. Palmoplantar, erythematous or erythrosquamous manifestations as well as pustules are typical (Fig. 1). It is not the underlying disease that is treated with the drug that is important. Why a TNF antagonist, which is normally very useful against psoriasis, can trigger this reaction is largely unknown. From a management standpoint, topical therapy with steroids is initially indicated; if the intensity is increased, TNF antagonist suspension is also indicated. It strongly depends on the intensity of the paradoxical psoriasis. If the intensity is mild, it can be discussed whether to switch to another TNF antagonist, which has been successfully practiced in some cases. In other cases, however, paradoxical psoriasis may manifest itself so severely that a switch to another substance class (e.g., ustekinumab) makes sense.

Anti-TNF-α-induced lupus
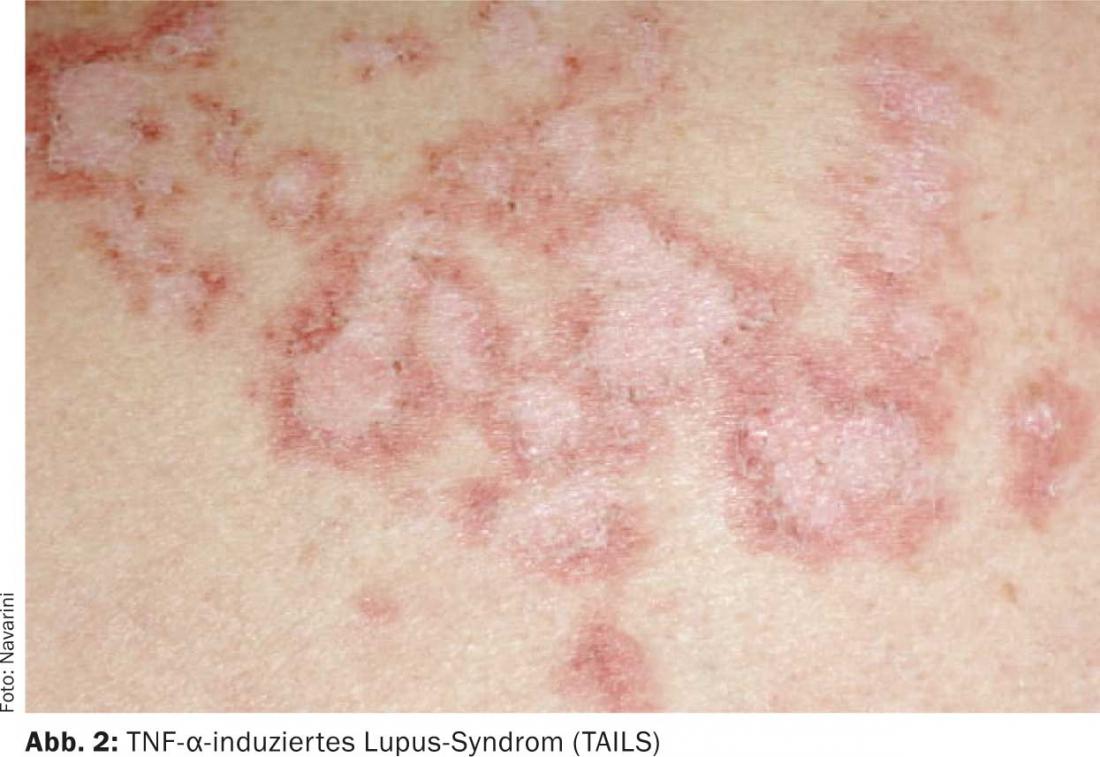
Another specific side effect of TNF antagonists is the so-called TNF-α-induced lupus syndrome (TAILS) (Fig. 2). This also occurs in approximately 0.1-0.5% of all cases. It is associated with the induction of antinuclear antibodies as well as skin manifestations consistent with lupus erythematosus. There are erythematous macules in the sense of butterfly erythema on the face, photosensitive exanthema in the décolleté area and on the rest of the body, and optionally pernions (nodular or papular, bluish swellings over the acra).
To date, there is no laboratory test that can unequivocally confirm the diagnosis, therefore we rely on the association of treatment with a TNF antagonist with the appearance of lupus symptoms. Therapy involves discontinuation of the TNF antagonist and administration of systemic steroids and hydroxychloroquine. Within three weeks to six months, the symptoms of TAILS are regressed. In the presence of TAILS, we tend not to switch to another TNF antagonist.
Neoplasia
In the literature, the topic of induction of neoplasia by biologics, especially TNF antagonists, is controversial. Cancer rates are clustered in patients with inflammatory systemic diseases such as psoriasis or rheumatoid arthritis compared to the normal population, with and without biologics treatment. When these patients are treated with TNF antagonists, the risk of epithelial skin tumors and melanoma is further slightly increased. All other cancers do not cluster compared to patients with the same diagnosis treated without biologics.
We recommend regular monitoring of the entire integument in all patients treated with biologics. In addition to the slightly increased risk of skin cancer due to the drug, an even more important effect has to be considered: A prior, often long-term phototherapy in psoriatics, which can also induce skin tumors as a long-term side effect.
The so-called unmasking effect of effective treatment with biologics in psoriatics must also be taken into account: Only after the disappearance of the psoriatic plaques, sometimes an actinic keratosis or a spinocellular carcinoma can be identified as a residual finding, which so far remained hidden in the sea of erythrosquamous changes.
Tuberculosis
Any treatment with TNF antagonists or the interleukin-12/23 antagonist ustekinumab should be preceded by a check to determine whether the patient has chronic infections. We have described the procedure elsewhere [1,2]. The reason for these mandatory screenings is the increased risk of reactivation of otherwise granulated tuberculosis. In recent years, several fatal cases of miliary tuberculosis have occurred, particularly in the southern regions of Europe. It should be noted that in the majority of these cases the initial focus was not in the lungs but in the gastrointestinal tract. Therefore, an x-ray alone is not an effective screening, but we recommend performing the quantiferon test or other interferon release tests. In addition, there is a twofold increased risk of new tuberculosis infection with TNF antagonist therapy. Therefore, patients traveling to high-risk areas should be screened periodically for reinfection.
Hepatitis and HIV
The guidelines we use also recommend screening for hepatitis B and C prior to biologics treatment. Studies have clearly shown that hepatitis B can be reactivated under TNF antagonists. The situation is less clear for hepatitis C and HIV. We screen for these infections to have a complete picture of any conditions that could potentially complicate the situation in the future. However, according to the current state of knowledge, no direct worsening of these infections is to be expected during biologics treatment.
Heart failure
It has been shown that treatment with TNF antagonists can lead to worsening heart failure when the drugs are administered at higher doses than normally prescribed. Therefore, we treat patients with heart failure NYHA III or IV resp. an ejection fraction below 50% not with TNF antagonists. In addition, if new heart failure develops, the TNF antagonist should in all likelihood be stopped.
Neurological diseases
Assuming that TNF-α is an important factor in the pathophysiology of multiple sclerosis (MS), studies have been conducted on the treatment of this disease with TNF antagonists. These studies clearly show a worsening of the disease, furthermore some patients got paresthesias and visual disturbances under etanercept or infliximab. Therefore, existing MS is a contraindication to treatment with TNF antagonists. We also perform an extended family history in each case. However, sporadic cases of MS in the family need not automatically constitute a contraindication to TNF antagonist treatment. No such neurologic drug side effects have been described with ustekinumab.
Rare cutaneous reactions to biologics
Urticaria and angioedema have been described in response to TNF antagonist therapy, and cutaneous sarcoidosis has been found in rare cases (0.04% of all patients). Eczematous reactions occurred with both TNF antagonists and ustekinumab. Another rare manifestation on the skin is lichen planus, which has been well documented in some patients taking TNF antagonists. Very rare side effects of TNF antagonists are alopecia areata, alopecia universalis and especially some cases of scarring alopecia.
Laboratory Abnormalities
When treating patients with biologics, which are generally well tolerated, the question often arises as to whether laboratory tests are necessary at all. We recommend these at intervals of three months, since we see the patients anyway on the occasion of the necessary determination of the “Psoriasis Area and Severity Index” (PASI). Furthermore, it must be taken into account that in the vast majority of cases these patients are not completely healthy, but that a significant percentage are multimorbid and suffer from metabolic syndrome. Therefore, any emerging problems should be identified at an early stage. However, there are few systematic studies on this.
We recently found clinically relevant eosinophilia in three patients under TNF antagonists in the course of these controls, which reduced again after switching to another drug within the substance class. Also, liver elevations frequently occur, which need to be further clarified – e.g., with concomitant low-dose methotrexate therapy to prevent induction of autoantibodies against the biologic.
Outlook
The substance class of biologics has enabled great successes in dermatology; therapy with biologics is a positive experience in the vast majority of cases, both for the treating clinicians and for the patients. Biologics treatment can be safely administered, particularly if the treating clinicians have a good level of training regarding any expected cutaneous side effects.
Biologics treatments are on the horizon for a host of other serious dermatological conditions. For example, belimumab (Benlysta®) is already registered and available for systemic lupus erythematosus, and apart from local injection reactions, no rare cutaneous side effects have yet been described. We assume that this is related to the currently still low number of patients who have received the drug over a longer period of time. Recently, it was also published in NEJM that dupilumab is very effective in atopic dermatitis [3].
In summary, we are looking at a great future in dermatology with specific biologic drugs, the majority of which have an encouraging benefit-risk profile. Our task is to use them skillfully and to recognize and effectively treat the specific and sometimes novel side effects.
CONCLUSION FOR PRACTICE
- By far the most common side effects of biologics are injection and infusion reactions.
- Paradoxical psoriasis occurs in approximately 0.5% of all patients treated with a TNF antagonist.
- Another specific side effect of TNF antagonists, TNF-α-induced lupus syndrome (TAILS), forces discontinuation of the TNF antagonist.
- Patients on TNF antagonists have a slightly increased risk of epithelial skin tumors and melanoma. All other cancers are not clustered.
- Prior to any treatment with TNF antagonists or ustekinumab, check whether the patient has chronic infections (tuberculosis, hepatitis C/B, HIV).
- The authors recommend laboratory controls at three-month intervals when treating with biologics.
Literature:
- Recommendations for biologics therapies, Dermatologicahelvetica 2009.
- Recommendations for biologics therapies, Dermatologicahelvetica 2011.
- Beck LA, et al: Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. NEJM 2014; 371(2): 130-9. doi: 10.1056/NEJMoa1314768.
DERMATOLOGIE PRAXIS 2014; 24(5): 10-13