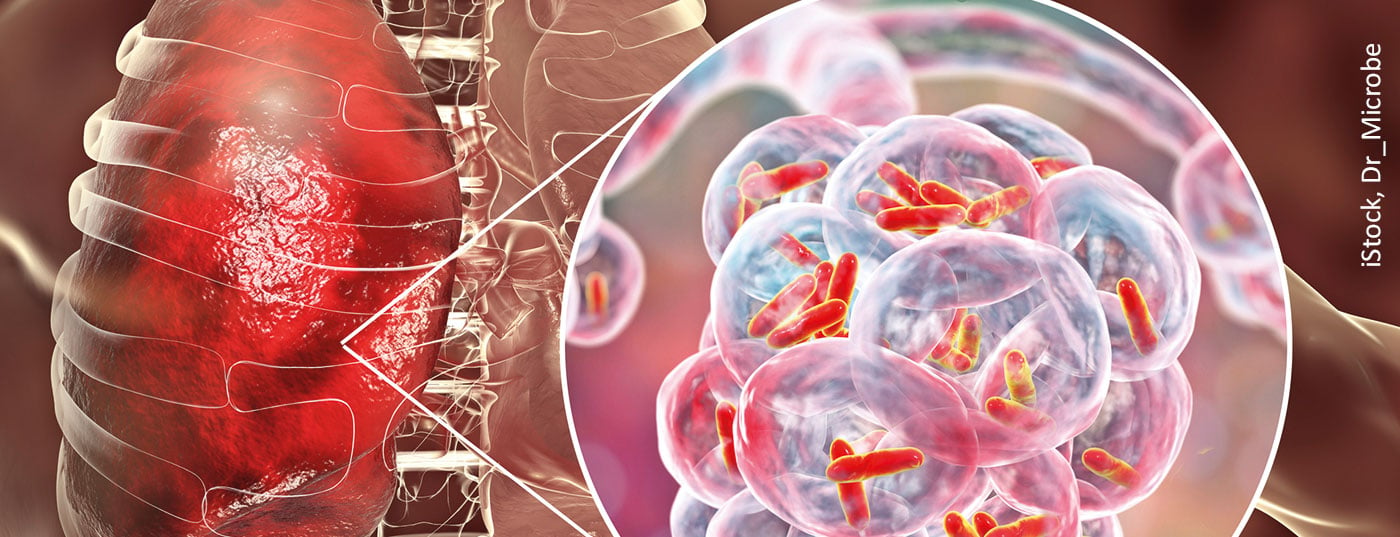
Tuberculosis is becoming increasingly rare in Switzerland, Austria and Germany. For example, only 4791 cases of tuberculosis were reported in Germany in 2019, an incidence of 5.8 new cases per 100,000 population. After a significant increase in 2015 as part of the wave of refugees, the numbers declined again for the first time in 2017 and stagnated in 2018. While 2020 shows a further decline, it is a special year due to underdiagnosis and masks disseminated during the Corona pandemic.
Tuberculosis is becoming increasingly rare in Switzerland, Austria and Germany. For example, only 4791 cases of tuberculosis were reported in Germany in 2019, an incidence of 5.8 new cases per 100,000 population. After a significant increase in 2015 as part of the wave of refugees, the numbers declined again for the first time in 2017 and stagnated in 2018. A significant decrease can be observed in 2019. Although 2020 shows a further decline, it is a special year partly due to underdiagnosis and partly due to the distancing and disseminated masks initiated in the context of the Corona pandemic [1,2].
The situation worldwide is completely different: According to estimates by the World Health Organization (WHO), around 10 million people worldwide contracted tuberculosis in 2018 and 1.5 million died from it. This makes tuberculosis one of the ten leading causes of death worldwide. The most commonly affected countries are India, Indonesia, China, Philippines, Bangladesh, Nigeria, Pakistan, and South Africa, which account for two-thirds of the world’s tuberculosis cases [1]. This also has an impact on Germany, Austria and Switzerland. For example, in recent years in Germany, the proportion of foreign-born tuberculosis patients has increased to 72% in 2019 [3].
As tuberculosis becomes rarer in Switzerland, Germany and Austria, the medical profession is losing knowledge of the disease and hygiene measures. This was different at the beginning of the last century. Thus, in his novel “The Magic Mountain”, set in Davos, Switzerland, Thomas Mann describes the various facets of the tuberculosis disease, but also the hygienic measures [4]. Thus, at each discharge, which not infrequently occurred after death, a thorough disinfection of the patient’s room was carried out, which we would call “final disinfection” today. What is interesting about this novel, published in 1924, is that no isolation of TB patients is described, nor are any measures taken against aerogenic transmission. There are no depictions of spacing rules, nor that masks or the like were worn. This is all the more astonishing because Robert Koch had already published Mycobacterium tuberculosis as the causative agent of tuberculosis in 1892. However, it exemplifies the focus on inanimate surface transmission in infectious diseases rather than human-to-human transmission. This way of thinking, common in infectious diseases, was also very popular at the beginning of the Corona pandemic and led to excessive disinfection measures, including the disinfection of writing utensils.
The aim of this article is to describe the necessary hygiene measures for tuberculosis based on current international recommendations and to identify measures that are not necessary.
Prevention in medical facilities
The greatest risk of infection comes from unrecognized and/or untreated cases of tuberculosis. Health care workers are at increased risk of transmission [5–7]. With the start of treatment, the contagiousness decreases rapidly. With recognition of a case of tuberculosis, the protective measures described below can be taken and the risk of infection greatly reduced. For this reason, the measures should already be applied in unclear cases or suspected cases of tuberculosis.
The patient’s cooperation is important for the implementation of hygiene measures. Therefore, special emphasis should be placed on individual education. The patient should always wear a mouth-nose protection when in contact with other persons. This effectively prevents the formation of aerosol as an infectious agent. In addition, a so-called “cough etiquette” must be observed. This means that the patient is instructed not to cough directly at anyone and, if necessary, to cover the mouth and nose with a paper towel while coughing and, if necessary, to dispose of coughed-up material containing pathogens in the waste containers provided and then to disinfect their hands.
Tuberculosis is not considered a highly infectious disease and is transmitted almost exclusively aerogenically via inhalation of minute droplet nuclei (aerosols <5 µm3). Infection usually occurs aerogenically, via minute droplets of bronchial secretion containing the pathogen, which are released by the ill person when coughing, sneezing, speaking or singing and are inhaled by contact persons. In contrast, larger droplet nuclei sediment more rapidly and can be eliminated by the self-cleaning of the respiratory tract. Their contagiousness is therefore rather low [7]. On inanimate surfaces, survival of Mycobacterium tuberculosis could be experimentally demonstrated up to 4 months [8], but this is a theoretical assumption, since the sedimented pathogens dried in sputum are unlikely to enter the lungs aerogenically in a relevant way [8,9]. In practice, this means that only patients with pulmonary tuberculosis who cough up appreciable amounts of the pathogen via bronchial secretions are considered to be infectious. In this case, detection is performed via sputum or bronchial secretions and microscopic direct detection, PCR or cultural detection. The Commission for Hospital Hygiene and Infection Prevention at the Robert Koch Institute (KRINKO) therefore logically classifies only infectious pulmonary tuberculosis, commonly referred to as “open”. Extrapulmonary tuberculosis can theoretically be spread through urine or pus, for example. However, this is very rare in practice, and extrapulmonary tuberculosis is therefore classified as noninfectious or “closed” tuberculosis [9]. The following hygiene recommendations therefore apply to infectious pulmonary tuberculosis or, in the context of surface disinfection, also to extrapulmonary tuberculosis, in which a spread of pathogens is to be feared.
Risk of infection
With the start of antituberculous therapy, pathogen excretion decreases rapidly and with it the risk of infection. At the latest 3 weeks after effective antituberculous therapy, the patient can no longer be assumed to be infectious. The response of the therapy can be read off well from the radiological course and improvement of the clinical picture, e.g. weight gain of the patient [9]. There is consensus in the WHO guidelines [10,11], the US CDC [12,13], the German Central Committee for the Control of Tuberculosis [7], and the UK NICE [14] that medical personnel and contacts to infectious tuberculosis should wear a respirator. FFP2 or N95 are unanimously recommended here. Both mask types are well known through the current Corona pandemic and need not be explained here. In addition, the UK recommendations recommend FFP3 masks for aerosol-generating processes, such as bronchoscopy, while for multidrug-resistant tuberculosis, or MDR-Tbc, all recommendations are consistent in calling for FFP3 masks. This recommendation is remarkable in that the transmissibility of Mycobacterium tuberculosis is not dependent on its drug resistance, thus the protective measures should actually be identical regardless of the resistance level. In fact, however, the protective measures here are made dependent on the severity of the secondary disease and the lack of treatability of multidrug-resistant tuberculosis, and the hygienic protection requirement is maximized. In addition to the filter class of the masks, it is essential that the personnel are trained in the use of the mask and are able to put it on and take it off hygienically. The correct sealing fit of the mask is also essential. During the corona pandemic, wearers are occasionally observed to have a high leakage rate due to their narrow face shape, which means that unfiltered air is inhaled next to the mask. The masks are thus essentially ineffective. A well-fitting mouth-nose guard would be a better choice in these cases. The fact that FFP masks are generally better than mouth-nose protection is thus one of the hygienic myths.
For the tuberculosis patient, all international recommendations advocate the wearing of a mouth-nose protection, as this measure effectively prevents the formation of an aerosol and spatial spread already mentioned above.
Room air technology
Currently, the issue of ventilation of rooms or the installation of a room ventilation system has again developed a new momentum. All the above-mentioned international recommendations contain the premise of a room ventilation system with negative pressure, if available, for patients with pulmonary tuberculosis in whose sputum bacteria can already be detected in the microscopic preparation, so-called “sputum-positive patients”. Few clinics and medical facilities treating infectious tuberculosis patients are likely to have such technology. Therefore, in Germany, for example, no room ventilation system (RLTA) is required for the treatment of even multidrug-resistant tuberculosis [9]. However, if a room ventilation system is available, it must be operated at negative pressure, i.e. the patient room must be at a relative negative pressure compared to the surroundings in order to prevent air from escaping into the neighboring rooms. Incidentally, a lock is not explicitly called for in any recommendation. If a room air conditioning system is used, the air must understandably be conveyed directly to the outside or back into the patient’s room via effective filtration. The main effect of a ventilation and air-conditioning system, apart from filtration, is to ensure regular exchange of air. Therefore, for window ventilation, both WHO and DZK state an air exchange rate of at least 2 times per hour. Window ventilation is ideally provided through opposing windows, where available. A Canadian study showed that when the air exchange rate was less than 2-fold per hour, the risk of tuberculin conversion, i.e., infection of medical personnel, tripled [15]. It is therefore essential to maintain this air exchange rate if no RLTA is available [6].
UV irradiation is increasingly used to destroy bacterial DNA. A wavelength of 294 nm is typically used here. The devices are used either directly in ventilation ducts or as transportable devices for final disinfection of patient rooms [6]. UV light irradiation should be seen as a supplement to existing measures and not as the sole measure. However, it is difficult to prove the effect of UV light as part of a bundle of measures in studies, which is why there is only limited evidence for its use to date and UV light has not yet found its way into the above-mentioned recommendations from the USA, the UK and Germany. Diel et al. therefore see their value as a complementary intervention option when adequate ventilation is difficult to implement [6].
Surface disinfection
As mentioned at the outset, surface disinfection has been introduced into hygiene for tuberculosis for more than 100 years. Despite this great popularity and distribution, the DZK classifies an aerogenic infection risk emanating from contaminated surfaces as very low, since sedimented particles are practically not re-released into the air as respirable droplet nuclei [7]. For this reason, no special disinfection of the patient’s room of a tuberculosis patient is propagated in the hospital during the inpatient stay [7,9]. On the other hand, experimental studies have shown that tuberculosis pathogens remain vital on inanimate surfaces for up to 4 months [8]. Both the German recommendations of the DZK and the KRINKO agree on a disinfection of the patient rooms during the stay of the tuberculosis patient, which does not differ from the other patient rooms [7,9,12,13]. The background to this, in addition to the described lack of risk of infection by already sedimented mycobacteria, is the fact that a significant reduction in the number of mycobacteria is achieved by the standard disinfectants after just a few minutes.
For functional areas, there is a lack of uniform recommendations so far. In analogy to patient rooms and other multi-resistant pathogens, such as MRSA, it can also be assumed here that disinfection of the surfaces close to the patient and, if necessary, additionally soiled surfaces is sufficient to prevent further spread of the pathogens [6,7]. The situation is different when the patient is discharged, where the aim is to completely eliminate all mycobacteria and at the same time, once the patient has been discharged, it is possible to ensure longer exposure times and higher concentrations in the room without endangering the patient. In this case, DZK, KRINKO and CDC recommend targeted disinfection with mycobacteria-active surface disinfectants [7,16–18]. It is important to observe the correct concentration and exposure time of the disinfectants used. Depending on the disinfectant used and the concentration selected, this can be 2 to 4 hours [19]. Room air disinfection, in which surface disinfectant is sprayed into the room air, is not required [7].
For the doctor’s office
If, for example, the presence of infectious pulmonary tuberculosis is suspected in a radiology practice during an X-ray examination, wipe disinfection of the surfaces with which the patient is suspected to have had contact must be carried out using a surface disinfectant that is ideally effective against tuberculosis. If this is not available, the available surface disinfectant should be used in any case. After drying, the room can be used again. However, if there is visible contamination with pathogen-containing material, e.g. sputum on the floor, targeted disinfection with a mycobacteria-active surface disinfectant must be carried out there [7,16 –18].
To date, there have been no studies that have shown a direct correlation of manifest tuberculosis disease and a contaminated area. Kramer already stated in 2006 “that despite theoretical long survivability of sedimented pathogens, they cannot be considered a relevant source of infection” [8]. Thus, there is no objective reason for the sometimes observed blocking of X-ray or endoscopy rooms for hours until the end of the exposure time of the surface disinfectants, after a patient with pulmonary tuberculosis (suspected) has stayed there. The same is especially true for patients with extrapulmonary tuberculosis, who are considered noninfectious and usually do not experience aerosol formation and therefore do not become infectious.
While respirators are generally recommended for infectious pulmonary tuberculosis, the wearing of protective gowns and disposable gloves requires a more nuanced approach. In the case of contact with patients with closed tuberculosis, if there is no contact with pathogen-containing material, e.g. wound secretion or urine, disposable gloves and protective gowns can also be dispensed with, as well as in the case of infectious pulmonary tuberculosis, if, for example, only a conversation is held with the patient. In these cases, so-called basic hygiene is sufficient, i.e. hand disinfection is performed before and after patient contact.
If there is a risk of contamination with pathogen-containing material, for example, during close contact with the infectious patient, bronchoscopies, endotracheal suction, sputum induction, or similar procedures, protective gloves and gowns must be worn [7,9]. Protective gowns are not to be confused with professional clothing such as a doctor’s gown. Protective gowns have the task of preventing work clothing from becoming contaminated with microorganisms and thereby endangering employees directly or other patients indirectly. These are long-sleeved, at least liquid-repellent gowns with back closure and closing cuffs at the arms, which can either be disinfected or disposed of as disposable gowns [9]. The protective gown is thus worn over the doctor’s gown or instead of the doctor’s gown. Interestingly, neither the DZK nor the KRINKO recommends wearing protective goggles [7,9]. Here, however, in case of doubt, protective goggles should be used for bronchoscopies and induced sputum.
Not part of the personal protective equipment in tuberculosis patients is the head hood. It is used to protect the patient against infection, for example during operations, but has no status as protective equipment for medical personnel in contact with tuberculosis patients. Transmission of tuberculosis via scalp or hair is not found in the literature. The same applies to transmission via footwear, so that shoe covers should be avoided at all costs. These only pose an unnecessary risk of accident, not only when trying to put them on while standing.
Summary and conclusion for practice
The most important measure to prevent infection by tuberculosis is early diagnosis of tuberculosis. A study from the Netherlands in the 1990s showed an average delay between first contact with a physician with typical symptoms and the diagnosis of tuberculosis of 2.5 months [20]. In the authors’ experience, this period is now more likely to have lengthened. In the case of contact with noninfectious, so-called “closed” tuberculosis, the measures of basic or standard hygiene are usually sufficient. In almost all cases of extrapulmonary tuberculosis, infectivity cannot be assumed.
In the case of infectious pulmonary tuberculosis, the wearing of an FFP2 mask by medical personnel is required, and the patient should wear a mouth-nose mask if possible, thus preventing the formation of an aerosol cloud and the spread of aerosols in the environment. With this measure, the risk of infection is significantly minimized. In addition, disinfection of patient contact surfaces is recommended for functional areas such as endoscopy and medical practices.
In case of close contact with the patient or if contact with infectious material is to be feared, the personnel should additionally wear a protective gown and disposable gloves. For the outpatient practice, in addition to standard hygiene, it is recommended that patients with suspected infectious tuberculosis who are undergoing outpatient treatment or diagnostics be admitted at the beginning or end of the consultation in order to avoid unnecessary contact with other patients. It is good if they can be immediately separated in one room. The patient should be encouraged to cough etiquette and wear a mouth-nose protection [9].
It is not uncommon for a diagnosis of tuberculosis to be made subsequently and for the patient to have visited the practice several times, for example, because of a loss of weight or a persistent cough. If tuberculosis is then diagnosed in the course of further clarification, disinfection measures are generally not necessary for past visits to the practice [9]. The same applies to the domestic sphere. There, too, the risk of infection ends with the diagnosis and the initiation of isolation measures. Extensive disinfection of the personal environment can be omitted. Only if immunocompromised persons or small children are in the household is surface disinfection with an agent effective against tuberculosis recommended.
Take-Home Messages
- The most important measure to prevent infection by tuberculosis is early diagnosis of tuberculosis.
- In the case of contact with noninfectious, so-called “closed” tuberculosis, the measures of basic or standard hygiene are usually sufficient. In almost all cases of extrapulmonary tuberculosis, infectivity cannot be assumed.
- In the case of infectious pulmonary tuberculosis, the wearing of an FFP2 mask by medical personnel is required, and the patient should wear a mouth-nose protection if possible.
- TB transmission via contaminated surfaces is unlikely. In practice, this means that wipe disinfection of the contact surfaces is sufficient and the room can be used again immediately after drying.
- For outpatient practice, in addition to standard hygiene, it is recommended that patients with suspected infectious tuberculosis be admitted at the beginning or end of the consultation to avoid unnecessary contact with other patients.
Literature:
- German Central Committee for the Control of Tuberculosis: www.dzk-tuberkulose.de.
- World Health Organization (WHO). Global TB report 2018. Geneva 2019.
- Report on the epidemiology of tuberculosis in Germany for 2019. Robert Koch Institute, Berlin 2020.
- Man Th: The Magic Mountain. S. Fischer Verlag Berlin 1924.
- Diel R, Niemann S, Nienhaus A: Risk of tuberculosis transmission among healthcare workers. ERJ Open Res 2018 Apr 9; 4(2): 2.
- Diel R, Nienhaus A, Witte P, et al: Protection of healthcare workers against transmission of Mycobacterium tuberculosis in hospitals: a review of the evidence. ERJ Open Res 2020; 6: 00317-2019; doi: 10.1183/23120541.00317-2019.
- German Central Committee for Tuberculosis. Infection prevention in tuberculosis – recommendations of the DZK. Pulmonology 2012; 66(05): 269-282.
- Kramer A, Schwebke I, Kampf G: How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases 2006, 6: 130; doi:10.1186/1471-2334-6-130.
- Recommendation of the Commission for Hospital Hygiene and Infection Prevention at the Robert Koch Institute; Infection prevention in the context of care and treatment of patients with communicable diseases, Bundesgesundheitsblatt 2015; 58: 1151-1170.
- World Health Organization. WHO policy on TB infection controling health-care facilities, congregate settings and households. Geneva: World Health Organization 2009.
- World Health Organization (WHO). WHO guidelines on tuberculosis infection prevention and control, 2019 update. Geneva 2019.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon R.: CDC Guidelines for preventing the transmission of Mycobacterium tuberculosis in healthcare settings, 2005. MMWR Recomm Rep 2005; 54: 1-141.
- Guideline for Disinfection and Sterilization in Healthcare Facilities 2008. Edits and Changes [February 2017]. Available at: www.cdc.gov/infectioncontrol/guidelines/disinfection/updates.html. Accessed August 25, 2019.
- National Institute for Health and Care Excellence. Tuberculosis: prevention, diagnosis, management and service organization (NICE guideline 33) 2016. Available at: www.nice.org.uk/guidance/ng33. Accessed August 25, 2019.
- Menzies D, Fanning A, Yuan L, et al: Hospital ventilation and risk for tuberculous infection in Canadian healthcare workers. Canadian Collaborative Group in Nosocomial Transmission of TB. Ann Intern Med 2000; 133: 779-789.
- Recommendation of the Commission for Hospital Hygiene and Infection Prevention at the Robert Koch Institute; Hygiene requirements for the cleaning and disinfection of surfaces, Bundesgesundheitsblatt 2004; 47: 51-61.
- Schulz-Stübner S: Tuberculosis in hospital hygiene, hygiene officer physician and ABS officer physician; Springer 2017: 341-346.
- CDC Guideline for Disinfection and Sterilization in Healthcare Facilities 2008.
- Disinfectants Commission in the Association for Applied Hygiene (VAH). Disinfectant list of the VAH. Wiesbaden: Mhp-Verlag GmbH 2011.
- Geuns van HA, Hellinga HS, Bleiker MA, Styblo K: Surveillance of diagnosis and treatment measures in the Netherlands. TSRU Program Report 1987; 1: 60-81.
InFo PNEUMOLOGY & ALLERGOLOGY 2021; 3(4): 12-16.