Around 80% of all anemias are iron deficiency anemias. In addition to malnutrition or malabsorption, there may be an increased iron requirement or iron loss due to chronic bleeding. If oral iron therapy does not prove effective, intravenous preparations are used for iron substitution. Nowadays, dextran-free iron complexes are preferred, which have only a low risk of an anaphylactoid reaction.
In anemia, the oxygen transport capacity of the blood is reduced, which jeopardizes an adequate supply of oxygen to the body. Typical symptoms include fatigue, dizziness, lack of concentration and nausea, reported PD Dr. med. Peter Staib, Chief Physician at the Clinic for Hematology and Oncology, St. Antonius Hospital, Eschweiler near Aachen [1]. Iron deficiency anemia can also lead to symptoms of the cardiovascular system, such as compensatory tachycardia or dyspnea, and in CHD** patients with pronounced anemia, an unstable situation is a possible consequence, the speaker explained [1].
** CHD = coronary heart disease
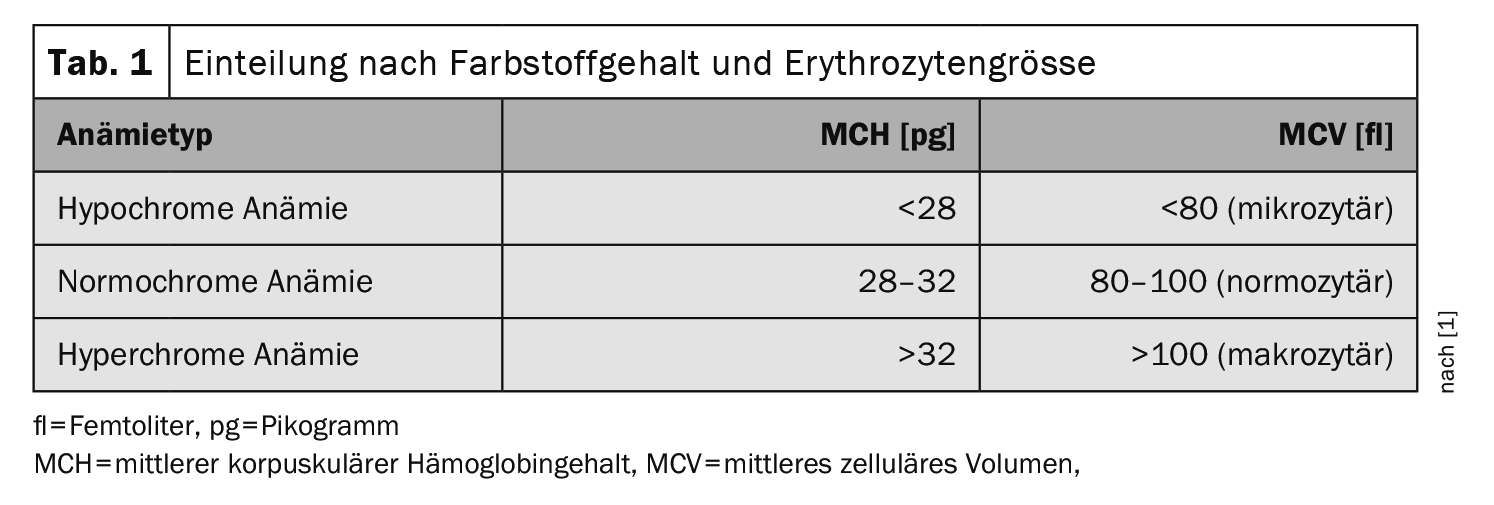
Anemia can be divided into five groups on the basis of reticulocytes, erythrocyte size, erythrocyte pigment content and hemolysis parameters [1]:
- Normochromic anemia
- Hypochromic anemia
- Hyperchromic anemia
- Hemolytic anemia
- Acute bleeding anemia
The most common form of hypochromic microcytic anemia (Table 1) is iron deficiency anemia. In microcytic anemia, the body does not produce enough hemoglobin (Hb), so the erythrocytes appear smaller than usual. In contrast to microcytic anemia, sufficient Hb is present in macrocytic anemia, but the erythrocyte count is reduced. In normocytic anemia, the amount of red blood cells and Hb match, but there are too few in total – as in bleeding anemia, for example. If the hemoglobin concentration falls below the age- or gender-specific normal value (12 g/dl for women and 13 g/dl for men) due to iron deficiency, iron deficiency anemia is present [2,3].
Ferritin, MCV, CRP: important parameters
First, a complete blood count including erythrocyte indices and reticulocyte production index (RPI) should be performed and, for the sake of completeness, a differential blood count with determination of CRP. In addition to the hemolysis parameters (bilirubin, LDH, haptoglobin, urobilinogen), the determination of ferritin and, if necessary, serum iron and transferrin saturation is essential. “Iron deficiency anemia is the most common and most important anemia,” explained Dr. Staib [1]. Iron is stored with the help of ferritin (box) . Hemosiderin is also involved in iron storage. This insoluble protein-iron complex, which consists of about 30% iron, is a degradation product of ferritin that can be detected microscopically in the macrophages of the bone marrow, liver and spleen. With a diagnostic panel consisting of ferritin, MCV and CRP, iron deficiency as the cause of anemia can be diagnosed with sufficient certainty in most cases [3].
| Ferritin is a water-soluble complex consisting of an outer protein shell (apoferritin) with a crystalline core of iron oxyhydroxide inside. Apoferritin can absorb up to 4500 iron oxyhydroxide molecules. Ferritin is found in all body cells as well as in body fluids. Its serum concentration correlates well with iron stores in healthy people, with 1 µg/l ferritin corresponding to 10 mg of stored iron |
| according to [3] |
Clarifying the causes of iron deficiency
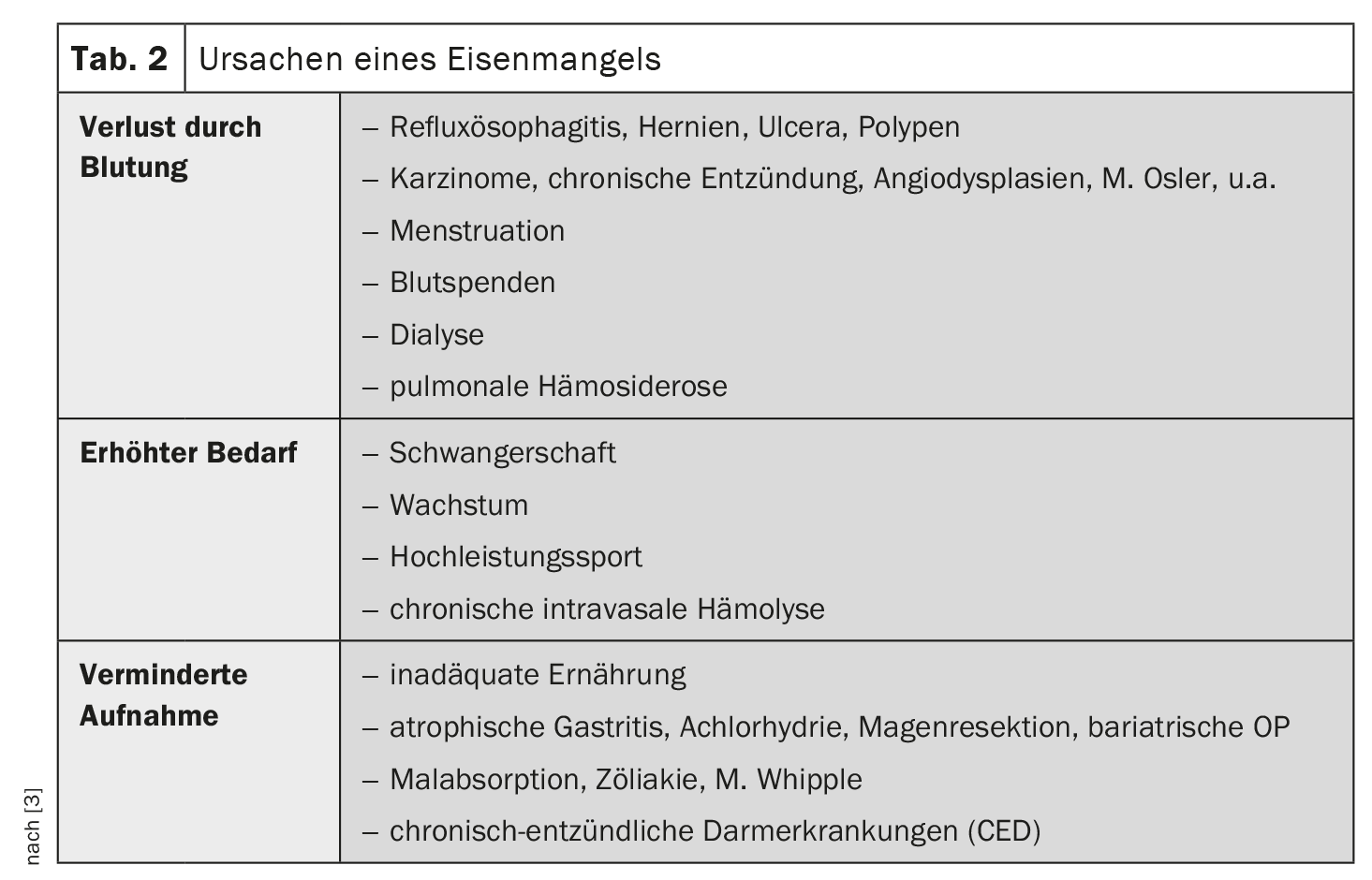
In the vast majority of cases, iron deficiency is caused by increased loss or consumption; absorption disorders are rare (Table 2). If iron deficiency is detected, malignant and chronic inflammatory diseases of the gastrointestinal tract must be ruled out first and foremost. The following clarification steps are recommended [3]:
- Medical history: diet, bleeding, medication, blood donations, infections, menstruation, operations, bowel movements, hemorrhoids
- Physical examination: inspection of the anal region, palpation of the abdomen, rectal digital examination
- Laboratory tests: Stool examination for occult blood
- Functional examinations: Gastroscopy, colonoscopy, sonography of the abdomen,
- Advanced diagnostics: MRI-Sellink, capsule endoscopy, enteroscopy, bronchoscopy.
The occult blood test is an established method for screening for gastrointestinal bleeding. However, the test is negative in about half of all patients with colon carcinoma. Therefore, endoscopic clarification by means of gastroscopy and colonoscopy is recommended to clarify chronic gastrointestinal bleeding if there is no other clear cause of iron deficiency. If blood is detected in the stool, a source of bleeding in the small intestine must be considered if the stomach and colon are normal, so that the diagnostic measures should be extended accordingly.
Indication for iron substitution?
Any iron deficiency that has reached the stage of iron-deficient erythropoiesis is an indication for iron administration. If possible, iron should be substituted orally. However, only 5-10% of the dose is absorbed, which must be taken into account when calculating the requirement. Numerous preparations are available for oral iron supplementation; bivalent iron preparations should be preferred. In order to optimize iron absorption, substitution every other day is being discussed [4]. It is recommended to continue oral iron therapy for at least three months after correction of the anemia so that the iron depots are also sufficiently replenished. If oral iron preparations are not tolerated, in patients with an iron absorption disorder or if oral medication is not sufficient or not tolerated, intravenous substitution should be used. The feared allergic and anaphylactic reactions to intravenous iron preparations are at least partly due to their carbohydrate content, with the previously used high-molecular dextran being particularly problematic. The dextran-free, intravenously administered iron preparations available today include
- Iron(III) gluconate complex
- Iron(III) hydroxide sucrose complex
- Ferric carboxymaltose
- Iron(III) derisomaltose
| Iron deficiency in heart failure Iron deficiency is associated with a poorer prognosis in patients with heart failure. This applies to both iron deficiency anaemia and pre-anaemic iron deficiency, because in both patient collectives, iron supplementation with iron carboxymaltose led to an improvement in quality of life, performance, NYHA class and a reduction in the risk of hospitalization [9]. Based on these studies, the ESC guidelines recommend the administration of iron carboxymaltose in patients with heart failure with a ferritin <100 µg/l, or with a transferrin saturation <20%, in order to improve performance and quality of life [10]. |
The newer iron formulations, such as iron carboxymaltose and iron(III)-derisomaltose, are becoming increasingly important in Europe. Thanks to their high stability, they allow the application of much larger single doses, so that iron deficiency can usually be corrected in a single session. These new formulations also have a high safety profile [5–8]. If administered intravenously too quickly, all iron preparations can overload the transferrin binding capacity and cause flushing symptoms due to the free, unbound iron. This side effect can be avoided by protracted administration, so that intravenous iron should preferably be administered as a short infusion. To be on the safe side, patients should be monitored during the infusion and a follow-up period of 30 minutes should be planned.
Congress: DGIM Annual Conference
Literature:
- “Anemia – What to do?”, PD Dr. med. Peter Staib, 130. Congress of the German Society of Internal Medicine (DGIM), 13.04.2024.
- “Iron deficiency”, Guideline, www.medix.ch/wissen/guidelines/eisenmangel,(last accessed 07.06.2024)
- Hastka J, Metzgeroth G, Gattermann N: Iron deficiency and iron deficiency anemia, Onkopedia guideline, July 2022. www.onkopedia.com,(last accessed 07.06.2024)
- Nielsen OH, Coskun M, Weiss G: Iron replacement therapy: do we need new guidelines? Curr Opin Gastroenterol 2016; 32: 128-135.
- Auerbach M, et al: A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J Hematol 2019; 94: 1007-1014.
- Blumenstein I, et al: Newer formulations of intravenous iron: a review of their chemistry and key safety aspects – hypersensitivity, hypophosphatemia, and cardiovascular safety. Expert Opin Drug Saf 2021: 20: 757-769.
- Bailie GR, Mason NA, Valaoras TG: Safety and tolerability of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Hemodial Int 2010; 14: 47-54.
- Fortuny J, et al: Use of intravenous iron and risk of anaphylaxis: A multinational observational post-authorization safety study in Europe. Pharmacoepidemiol Drug Saf 2021; 30: 1447-1457.
- Anker SD, et al: Ferric carboxymaltose in patients with heart failure and iron deficiency. Engl J Med 2009; 361: 2436-2448.
- McMurray JJ, et al: ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803-869.
HAUSARZT PRAXIS 2024; 19(6): 42-43 (published on 26.6.24, ahead of print)
InFo ONCOLOGY & HEMATOLOGY 2024; 12(3): 32-33