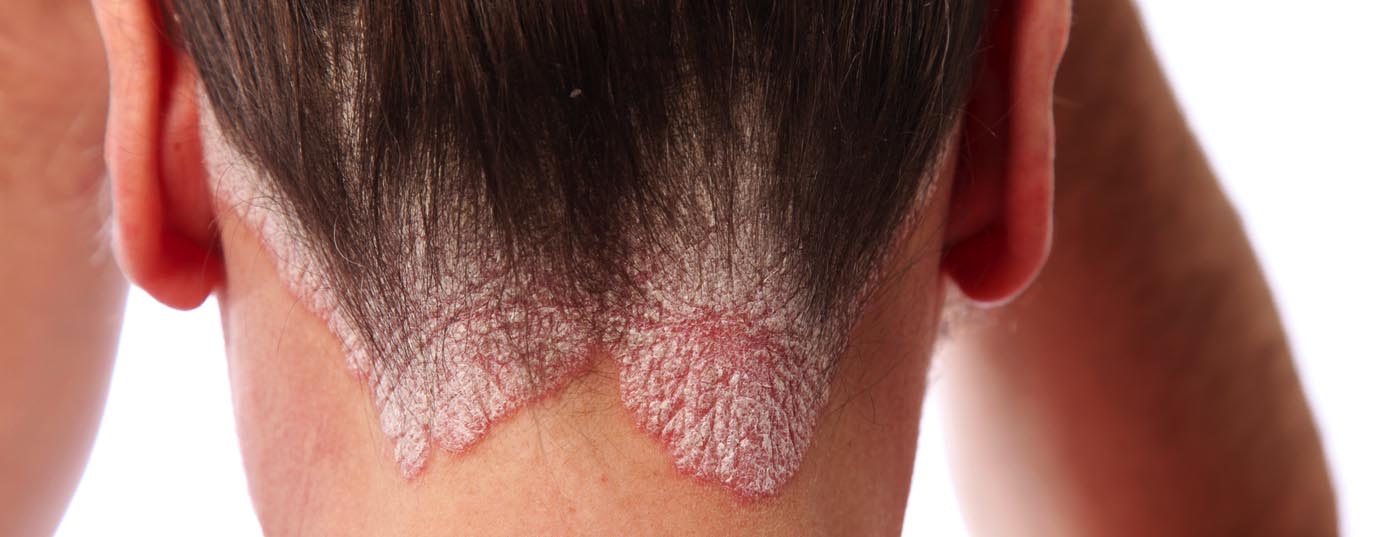
Children and adolescents with psoriasis are often significantly limited in their quality of life. There is a need for new treatment options for this patient population, especially for cases with moderate and severe psoriasis. In addition to ixekizumab, which recently received a marketing authorization extension in Switzerland for children and adolescents aged 6 to 17 years, secukinumab has also proven effective and safe in this patient group and has already received a marketing authorization extension in the EU.
Psoriasis occurs less frequently in children and adolescents than in adults, but the level of suffering is particularly high. Eliciting health-related quality of life is therefore also an important aspect of the medical history and assessment of findings (box) [1]. From a psychosocial point of view, stigma and discrimination can severely affect the psychological well-being of adolescent psoriasis patients. New therapeutic options for the treatment of moderate to severe plaque psoriasis are particularly in demand for this patient group. In the field of biologics, recent Phase III study results give cause for hope. The two interleukin (IL)17A antibodies, ixekizumab and secukinumab, which have long been an evidence-based and established treatment option in adults, have shown promising results. Ixekizumab (Taltz®) was approved in Switzerland in December 2020 for the treatment of moderate-to-severe plaque psoriasis in children and adolescents aged 6 years and older with a body weight of at least 25 kg in whom other systemic therapies have not been effective (review 1) [2].
|
“Children’s Dermatology Life Quality Index (CDLQI). According to the current s2k guideline for the treatment of psoriasis in children and adolescents, the PASI (Psoriasis Area and Severity Index) and BSA (Body Surface Area) scores commonly used in adults can be used to classify the severity of skin manifestations. The cut-off values for moderate to severe psoriasis are identical (BSA >10 or PASI >10). The Children’s Dermatology Life Quality Index (CDLQI) exists for assessing quality of life in children and adolescents under 16 years of age. This questionnaire is used in clinical trials as well as in routine care and is validated for this age group [1,5]. The cut-off value for severe psoriasis is also 10. In patients aged 16 years and older, the DLQI also used in adults can be used. Consideration of health-related quality of life is of great importance for the medical care of children and adolescents with psoriasis and should be included in treatment decisions. It is not possible to infer the extent of impairment in quality of life from the severity of skin manifestations; there is evidence that CDLQI scores correlate only moderately with PASI and PGA scores [1,6]. |
Secukinumab soon to be approved alongside ixekizumab?
For adults with moderate to severe plaque psoriasis, secukinumab (Cosentyx®) has long been an evidence-based and established treatment option. That the IL17A inhibitor is also effective and safe in children and adolescents is shown by new data presented at the EADV Annual Meeting 2020 by Prof. Christine Bodemer, MD, from Hopital Becker-Enfants Malades, Paris (F) [3]. In a phase III study, the IL17A inhibitor showed efficacy sustained until week 52 in patients aged 6 to <18 years with severe plaque psoriasis, leading to both a reduction in skin manifestations and an improvement in quality of life. PASI90 response rates were significantly higher compared to etanercept and secukinumab also performed better than etanercept with respect to PASI75.

A total of patients aged 6 to <18 years with severe plaque psoriasis were randomized to the trial conditions secukinumab low-dose (n=40), secukinumab high-dose (n=40), etanercept (n=41), or placebo (n=41). The dosing regimen was weight stratified. Patients with a body weight up to 50 kg received 75 mg in the low dose (LD) and those with a body weight of ≥50 kg received 150 mg. In high dosage (HD), the following regimen was used: <25 kg,75 mg; 25-50 kg, 150 mg; ≥50 kg, 300 mg. At both high and low doses, secukinumab proved significantly superior to placebo in terms of PASI90, PASI75, and IGA at week 12. PASI75 response rates were 80.0% (HD) and 77.5% (LD), respectively, under secukinumab compared to 14.6% under placebo; in terms of PASI90, these values were 72.5% (HD) and 67.5%, respectively, for secukinumab compared to 2.4% under placebo. Efficacy persisted at both doses (HD, LD) through week 52, with secukinumab achieving higher response rates than etanercept for both PASI75, PASI90, PASI100, and IGA. PASI75 response rates were 87.5% (HD) and 87.5% (LD), respectively, under secukinumab versus 68.3% under etanercept. Regarding PASI90, the corresponding values were 80.0% (HD) and 75.0% (LD) vs. 51.2% in the etanercept group, respectively, and regarding PASI100 for secukinumab, 47.5% in the high dose (HD) and 40.0% in the low dose compared to 22.0% under placebo. Secukinumab also performed better than placebo and etanercept in terms of quality of life (CDLQI 0/1) at weeks 12 and 52. The safety profile of secukinumab was consistent with that of previous phase III studies in adults, and no new safety signals were reported. The incidence of adverse effects was comparable in the high dose as in the low dose. The most common side effects were nasopharyngitis, pharyngitis, and headache.
“Children’s Dermatology Life Quality Index (CDLQI).
According to the current s2k guideline for therapy of psoriasis in children and adolescents, the PASI (Psoriasis Area and Severity Index) and BSA (Body Surface Area) scores commonly used in adults can be used to classify the severity of skin manifestations. Cut-off values for moderate to severe psoriasis are identical (BSA >10 or PASI >10). The Children’s Dermatology Life Quality Index (CDLQI) exists for assessing quality of life in children and adolescents under 16 years of age. This questionnaire is used in clinical trials as well as in routine care and has been validated for this age group [1,5]. The cut-off value for severe psoriasis is also 10. In patients aged 16 years and older, the DLQI also used in adults can be used. Consideration of health-related quality of life is of great importance for the medical care of children and adolescents with psoriasis and should be included in treatment decisions. The severity of skin manifestations cannot be used to infer the extent of impairment in quality of life, for example, there is evidence that CDLQI scores correlate only moderately with PASI and PGA scores [1,6].
Source: EADV Annual Meeting 2020
Literature:
- AWMF: s2k guideline for therapy of psoriasis in children and adolescents, 2019, www.awmf.org/uploads/tx_szleitlinien/013-094l_S2k_Therapie-Psoriasis-Kinder-Jugendliche_2019-07.pdf
- Swiss Drug Compendium, www.compendium.ch/news/22487
- Bodemer C: Secukinumab demonstrated high efficacy and a favorable safety profile in pediatric patients with severe chronic plaque psoriasis: One-year results. Prof. Christine Bodemer, MD, EADV Annual Meeting, On-demand sessions, Oct. 29, 2020.
- Informedhealthonline.org: www.gesundheitsinformation.de (last accessed Feb. 17, 2021).
- Lewis-Jones MS, Finlay AY: The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 1995; 132: 942-949.
- de Jager ME, et al: A cross-sectional study using the Children’s Dermatology Life Quality Index (CDLQI) in childhood psoriasis: negative effect on quality of life and moderate correlation of CDLQI with severity scores. Br J Dermatol 2010; 163: 1099-1101.
DERMATOLOGIE PRAXIS 2021; 31(1): 26-27 (published 2/22/21, ahead of print).