Not only the quality of life, but also mobility and inhibition of progression in multiple sclerosis can be sustainably increased thanks to the latest therapeutic approaches. Immunomodulatory drugs have already proven their efficacy in everyday practice.
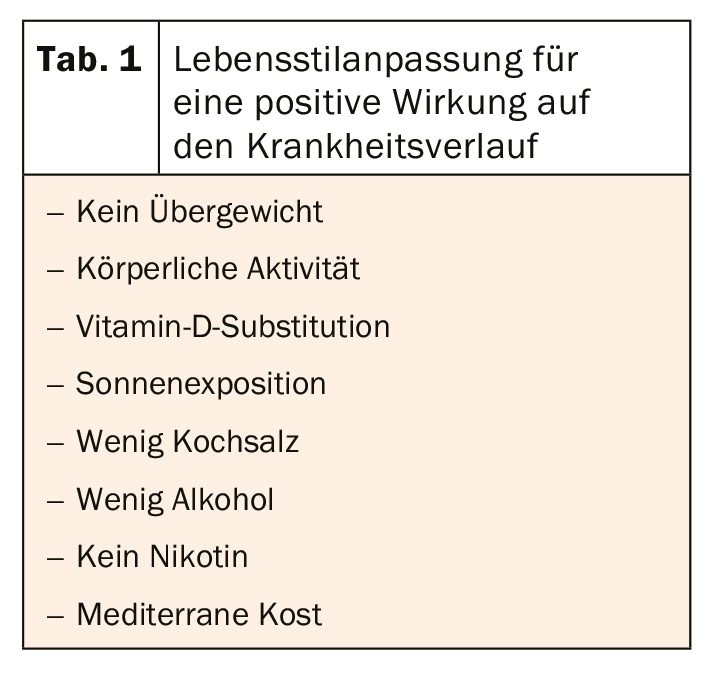
“Today, a good life despite MS is no longer a fiction,” Prof. Judith Haas, MD, Berlin, Germany, is certain. It is true that those affected are still concerned about their mobility, family planning and possible restrictions in their lifestyle or occupation. But more than half of all people with MS are still able to walk more than 500 meters after 27 years of disease, Haas said. Surveys also show that more than half of those affected do not experience any restrictions in their everyday working lives as a result of the disease. Only 28.3% have to interrupt or give up their work. The course of the disease can also be positively influenced by adjusting the lifestyle (Tab. 1). “As far as quality of life is concerned, we must not forget that patients usually assess it on a different basis than we doctors do,” the expert emphasized. While the physician focuses primarily on physical limitations, patients tend to focus on emotional impairments, role problems, or cognitive disadvantages that diminish quality of life. “Therefore, treatment of fatigue and depression also has a critical impact on quality of life,” Haas said.
Immunotherapies convince
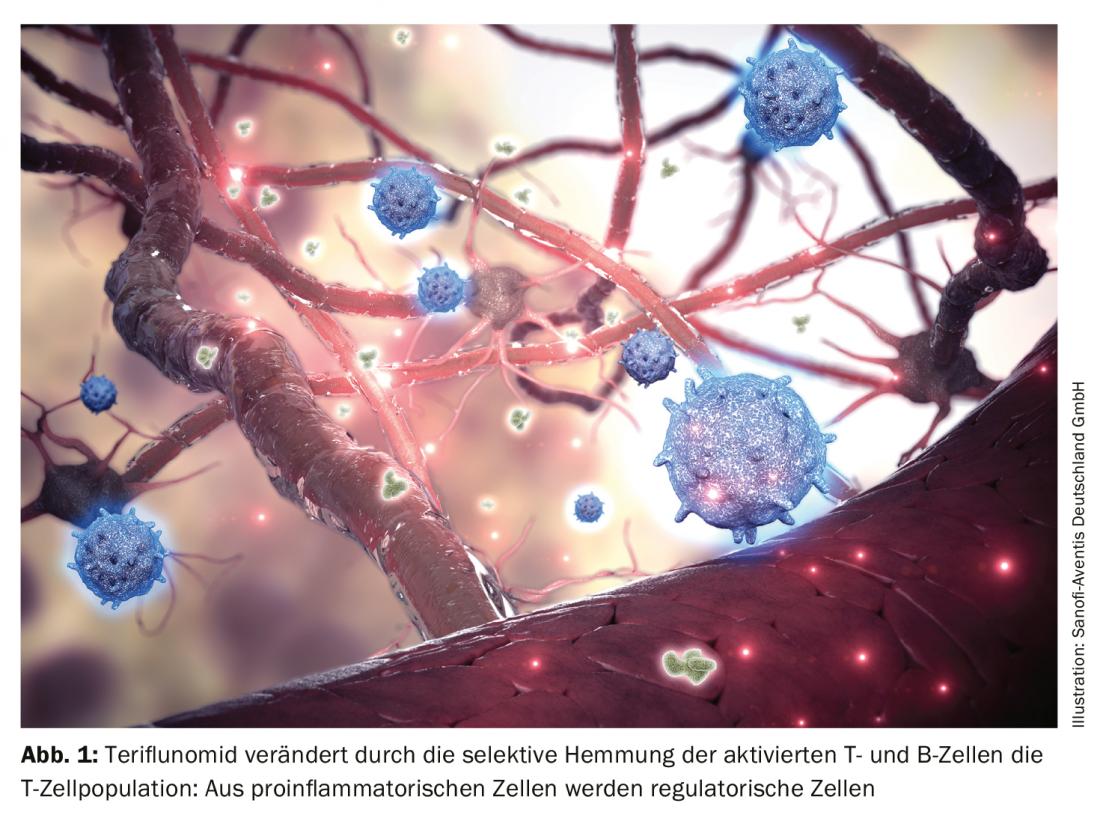
Immunomodulatory drugs such as teriflunomide (Aubagio®) have played a key role in the positive development of disease control in relapsing-remitting MS (RRMS). The drug selectively and reversibly inhibits the enzyme dihydroorotate dehydrogenase (DHODH) in mitochondria, thereby preventing the de novo synthesis of pyrimidine in activated lymphocytes. As a result, especially the activated CD4+ and CD8+ T cells relevant in the pathogenesis of MS are eliminated, as Prof. Dr. med. Sven Meuth, Münster, explained (Fig. 1). Results from real-world studies now demonstrate an efficacy of the immunomodulator comparable to that of dimethylflumarate (DMF) with regard to the mean relapse rate and the risk of progression. “However, significantly fewer adverse events occurred in the teriflunomide group,” the expert reported. Furthermore, when patients switched from prior therapy to teriflunomide, treatment satisfaction also increased significantly.
The humanized IgG1k monoclonal antibody alemtuzumab (Lemtrada®) has proven to be similarly effective. Current 8-year data demonstrate a low mean annual relapse rate and improvement in mental and physical well-being in pretreated patients. “In addition, at least 70% of patients had a stable or improved EDSS score each year compared with baseline,” Meuth said. An interim evaluation after six years also showed that only a proportion of 3.7% met the criteria for SPMS (secondary progressive MS) – in contrast to the comparison group of the MSBase registry, where it was 18%. Possible side effects, such as infusion-related reactions, mild to moderate infections or secondary autoimmune events, are detected at an early stage by a time-limited monitoring program and can be treated accordingly.
Source: Press workshop “5th MS Special(ists)”, June 25, 2019, Hamburg (D). Organizer: Sanofi
InFo NEUROLOGY & PSYCHIATRY 2019; 17(5): 34.