Venereal proctitis is not uncommon. 34% of HIV-positive men have anorectal problems [1]. Patients with proctitis symptoms and/or receptive anal intercourse in any form must always be thought of as having a sexually transmitted infectious disease (STI). Proctitis is frequently (>85%) asymptomatic with gonorrhea and Chlamydia trachomatis, serovars D-K [2]. Direct pathogen detection (bacterial culture, targeted PCR) from an anoscopically taken smear is suitable for clarification. Serological testing is only useful and indicated for syphilis diagnosis. HIV transmission is facilitated in proctitis [3]. Co-infections with other STIs should also be considered (including testing for HIV, hepatitis B, C). For lymphogranuloma venereum (LGV), “old disease in a new dress” applies – also in Switzerland [4].
Proctitis is defined as inflammation of the distal portions of the rectum. Infectious proctitis can be transmitted sexually during genito-anal and oro-genital contacts and also as a smear infection with fingers and instruments from a body orifice to the anus. The most common transmitted anorectal pathogens include N. gonnorhoeae, C. trachomatis (including lymphogranuloma venereum), herpes simplex virus, and Treponema pallidum [5]. Condom use does not guarantee protection against STI (“sexually transmitted infections”), as routes of transmission other than penile penetration are often involved [6].
Symptoms
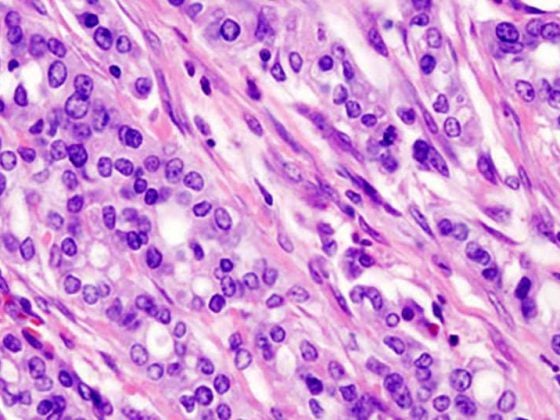
Possible symptoms of proctitis include mucopurulent fluoride, anorectal bleeding, constipation, urge to defecate, tenesmus, dull pain, rectal distension, or incomplete defecation (Fig. 1) . However, proctitis is also frequently asymptomatic (gonorrhea and chlamydia).
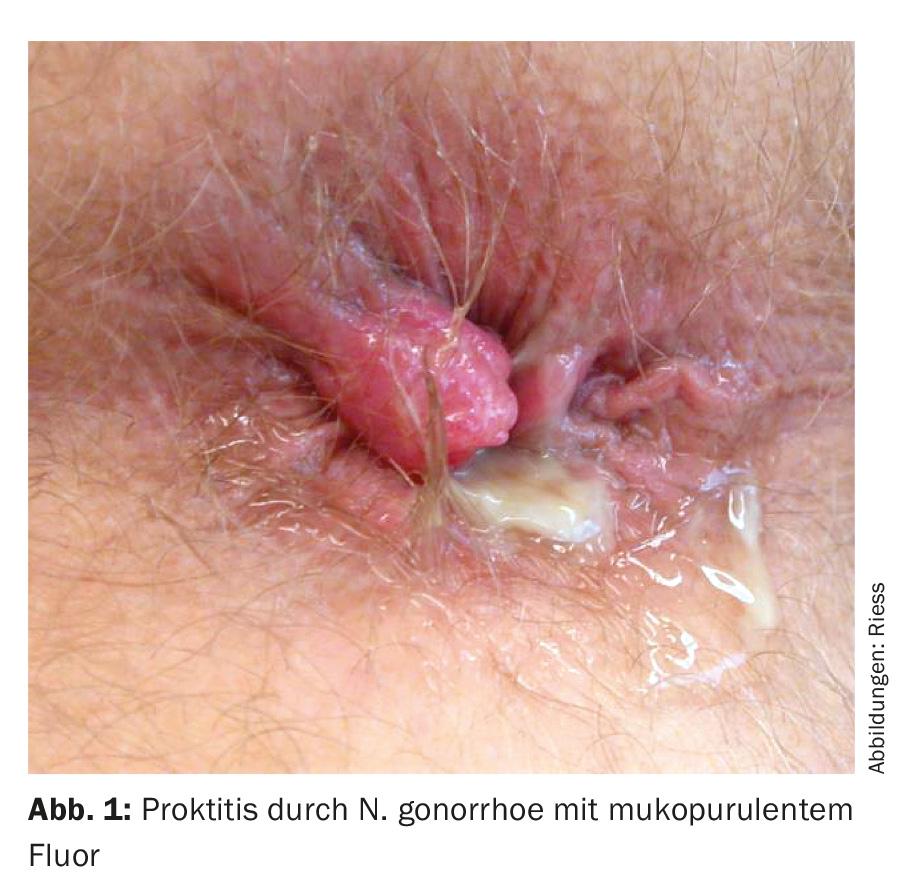
In proctocolitis , symptoms include small-volume diarrhea, blood in the stool, lower abdominal pain, abdominal tenderness, and a sensation of incomplete defecation. Transmission can occur through oro-anal sexual practices. When traveling, amoebae and, in HIV-positive patients, shigella can often be the cause of proctocolitis, so a stool examination is recommended if these symptoms are present.
Enteritis presents with large-volume, watery diarrhea, blood in the stool, mid-abdominal pain, nausea, vomiting, malaise, fever, and weight loss. The rectal mucosa is usually not affected. Shigellosis can be transmitted by oro-anal contact and cause these symptoms (Table 1).
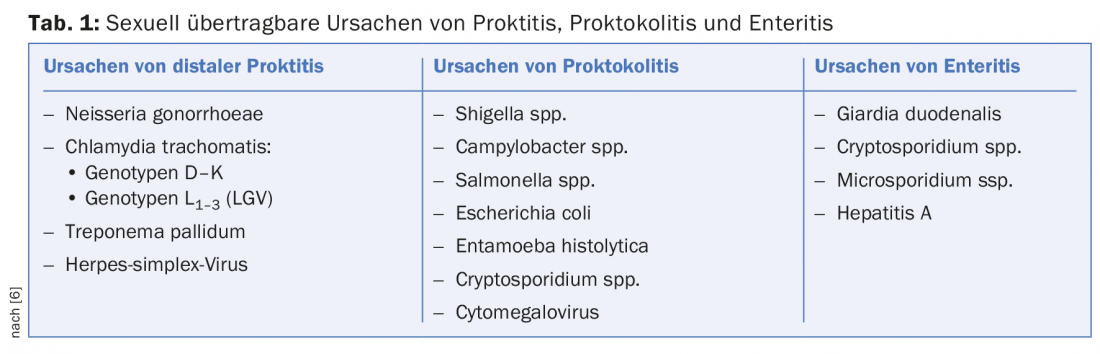
Examination for proctitis
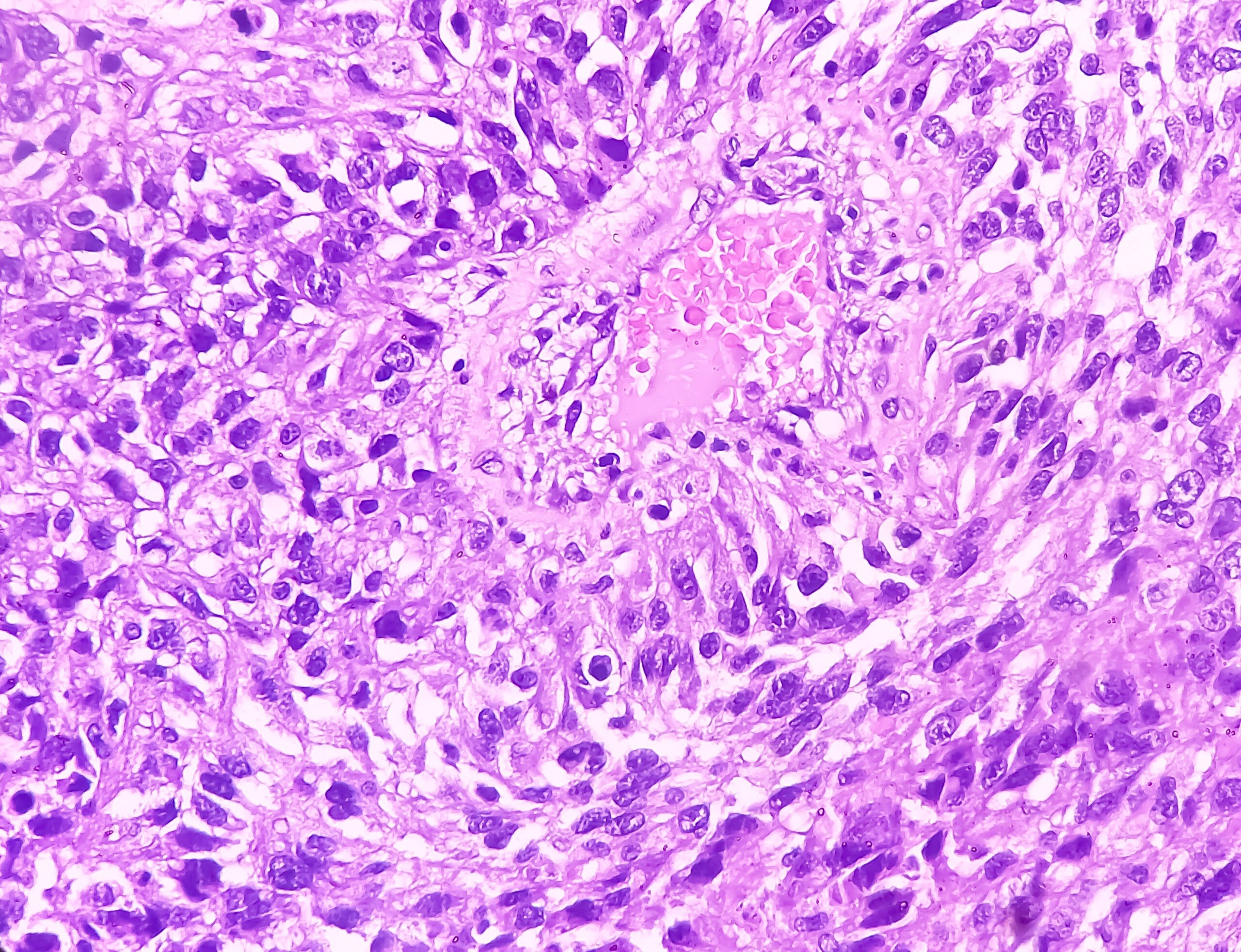
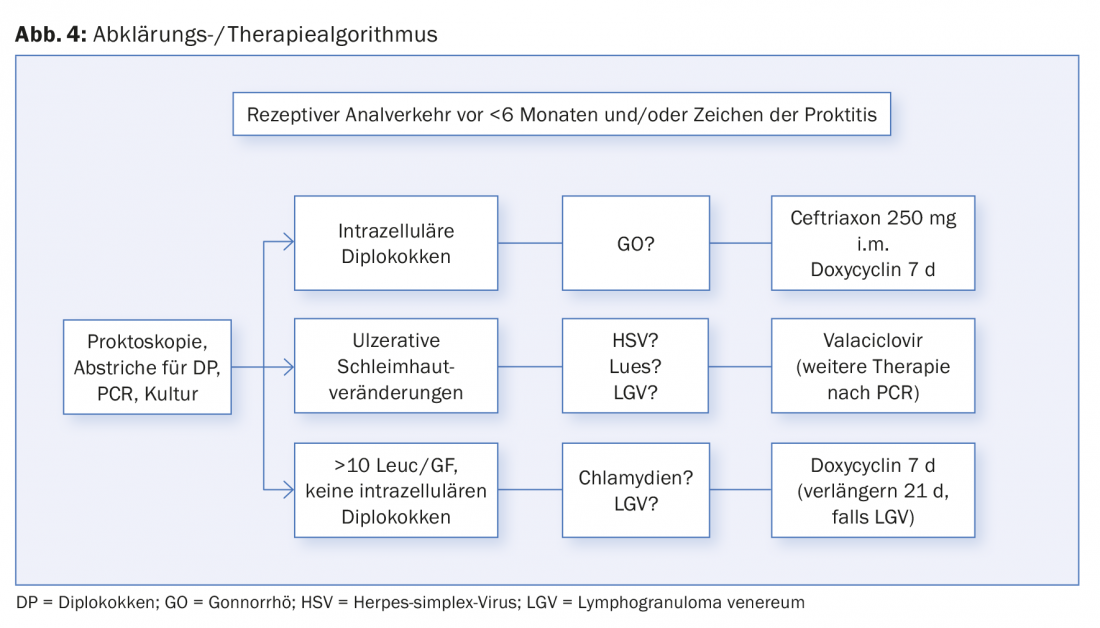
Proctoscopy is the examination of choice. The presence of mucus or purulent fluorine in the rectum, loss of vascular drawing, mucosal edema, contact bleeding, and ulceration are assessed. In addition, a direct preparation for the detection of diplococci (gonococci) and samples for PCR examination can be taken specifically through the proctoscope. Because venereal proctitis is often asymptomatic, it is recommended that if there is a history of receptive (especially unprotected) anal intercourse, regular examinations and swabs be taken to rule out this disease (Fig. 2).
Neisseria gonorrhoeae
According to WHO estimates, gonorrhea is one of the most common STDs, with 106 million new cases every year. Anal gonorrhea occurs predominantly in MSM (“men who have sex with men”) with receptive anal intercourse. It can also be transferred to the anal canal via insertion of fingers or instruments. It is often asymptomatic, but in florid proctitis there may be copious putrid secretions. Gonorrhea is diagnosed by PCR (polymerase chain reaction). However, because of frequent resistance, any PCR positive for gonococci should be followed by bacterial culture. With increasing resistance worldwide, dual therapy with 500 mg ceftriaxone i.m. and 2 g azithromycin p.o. is currently recommended [7].
Chlamydia trachomatis, serovars D-K
Serovars A-C of Chlamydia trachomatis cause trachoma (eye disease), and serovars D-K cause urethritis, cervicitis, endometritis, and proctitis. In Europe, infections caused by Chlamydia trachomatis, serogroup D-K, are the most commonly reported sexually transmitted infections [2,8,9]. Even more common than anal gonorrhea, anal chlamydial infection (serovars D-K) is also almost always asymptomatic. Clinically, however, findings of proctitis are often found. Detection is by PCR from a smear. Standard therapy is 100 mg doxycycline 2×/d for seven to ten days, alternatively azathioprine 1 g once.
Chlamydia trachomatis, serovars L1-3 or lymphogranuloma venereum (LGV).
Serovars L1-3 cause lymphogranuloma venereum, which is invasive, lymphotropic, and usually symptomatic. However, asymptomatic courses are also described [2,8]. Prior to 2004, this disease occurred predominantly in the equatorial region. Since then, an endemic occurrence of LGV has been observed in HIV-positive MSM in the Western world. A cluster of LGV infections was also observed in Zurich. As in the other European centers, HIV-positive MSM were predominantly affected [4]. Most common serovar type is L2b [10].
The incubation period is one to four weeks. The three-phase course begins with a genital ulcer or erosion (stage I), followed by inguinal lymphadenopathy (Bubo, stage II) and finally an ano-genito-rectal syndrome with fibrosis and irreversible complications (stage III). Complications may include fistulas, lymphatic obstruction, elephantiasis, strictures, or esthiomata (ulcerative disease of the female genitalia). In primary rectal infection, usually only the symptoms of proctitis are found. In contrast to anal infections with Chlamydia trachomatis, serovars D-K, LGV often results in severe proctitis, which is not infrequently misinterpreted as inflammatory bowel disease. Globally, LGV accounts for only 2-10% of genitoulcerative diseases, but it is on the rise [4,9]. LGV is the third most common cause of proctitis in MSM after HSV and gonorrhea [11].
For the detection of the serovar, PCR is considered the gold standard. Culture is obsolete and serology is nonspecific. According to the European consensus recommendation, therapy consists of doxycycline 2×100 mg/d for 21 days. There is too little evidence for the efficacy of a single dose of azithromycin. Alternatively possible is erythromycin 4×500 mg/d for 21 days.
Treponema pallidum
Since the turn of the millennium, syphilis has experienced a true renaissance. It occurs predominantly in men, especially MSM [7]. Like all other sexually transmitted diseases, it also promotes HIV transmission [3]. The primary effect is extragenital in over 20%. Primary anal lesions are usually asymptomatic, often discovered only as incidental findings during proctologic examinations. Clinically, ulcerative painless mucosal lesions or perianal ulcerations are found. In stage II of lues, condyloma lata, among others, occur and are highly contagious.
Detection of first-stage syphilis is by dark-field microscopy, although this is now usually replaced by the more sensitive and much simpler PCR assay. After about five weeks, reliable serological detection of infection (TPPA, VDRL, IgM) is achieved. The therapy is and remains a depot injection with benzathine benzylpenicillin once 2.4 million units intramuscularly. Currently, no resistances are found.
Herpes simplex virus
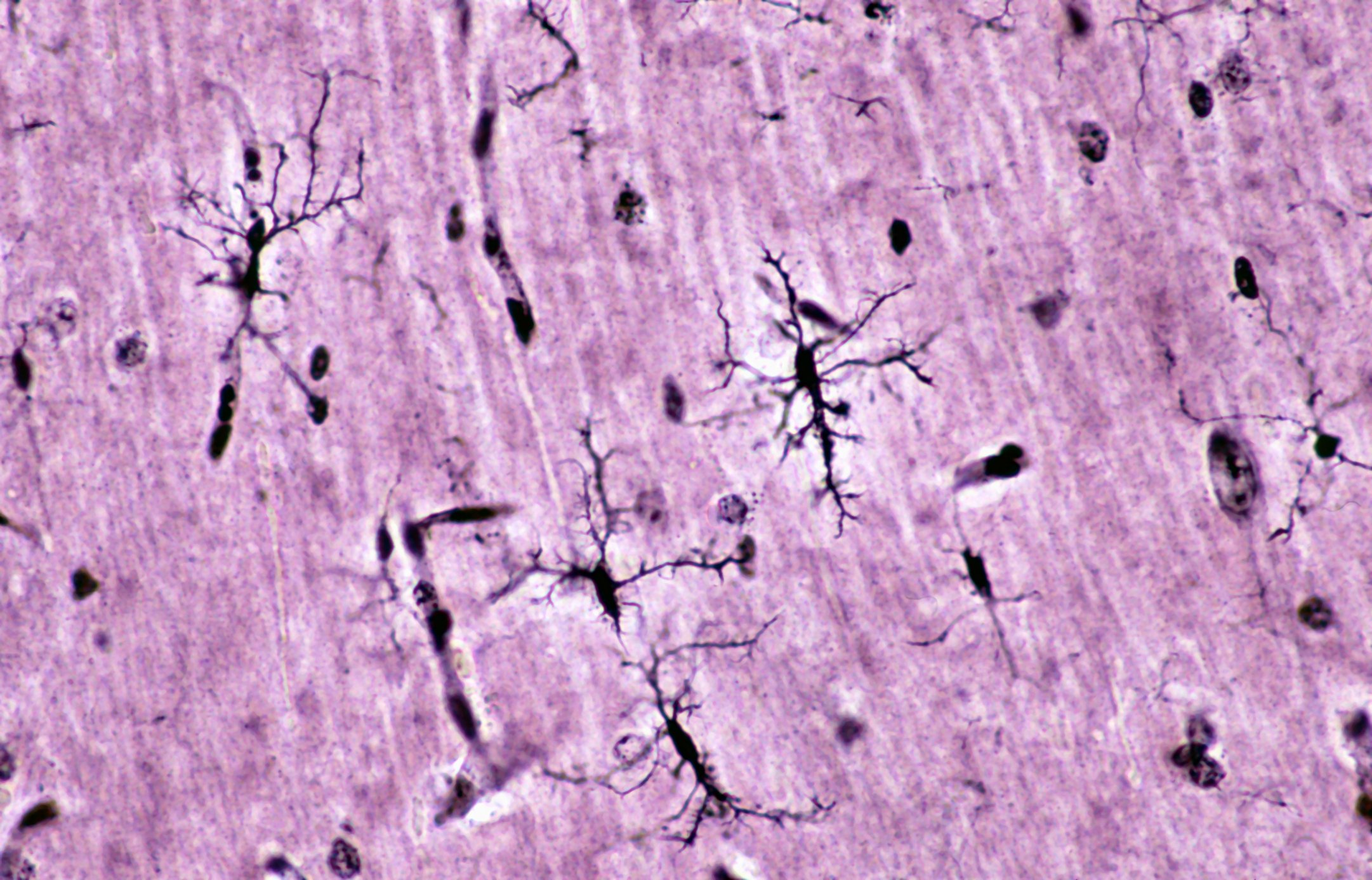
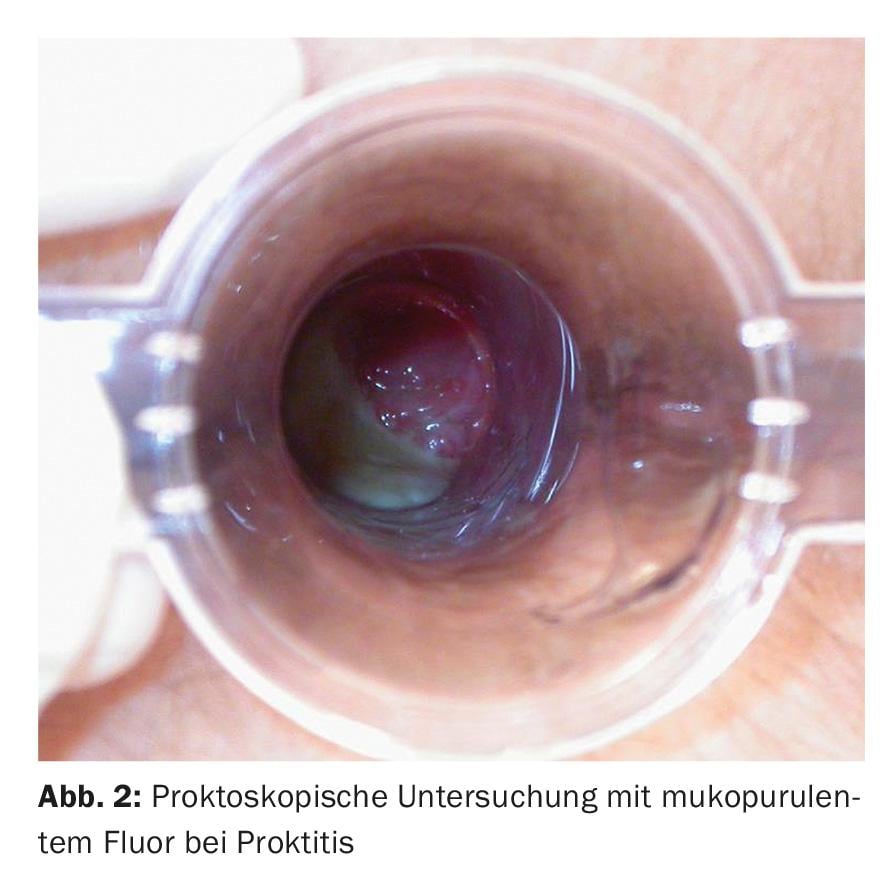
Genital herpes infections are caused by herpes simplex type 2 in more than 80%, with chronic recurrent courses in about 20% of cases. The characteristic clinic of grouped vesicles is usually absent in perianal localization, and atypical areal redness, ulcerative mucosal lesions, and perianal ulcers are seen (Fig. 3). Infestation is often associated with itching and pain. Necrotizing and ulcerative forms are seen in immunodeficiency. The detection is made by PCR. Therapy consists of valaciclovir 2×500 mg p.o. for five days.
Differential diagnoses
Differential diagnosis of proctitis must also include non-venereal causes of proctocolitis or enteritis with the appropriate pathogens (Shigella spp, Salmonella spp, Campylobacter spp, cytomegalovirus, amebiasis, giardiasis), rectal staphylococcal infection, and chronic inflammatory bowel disease.
In the case of perianal inflammatory or ulcerative skin changes, anal carcinoma, an epithelial tumor such as basal cell carcinoma, an irritative-toxic genesis, condylomata acuminata as well as Crohn’s disease must also be excluded as causes.
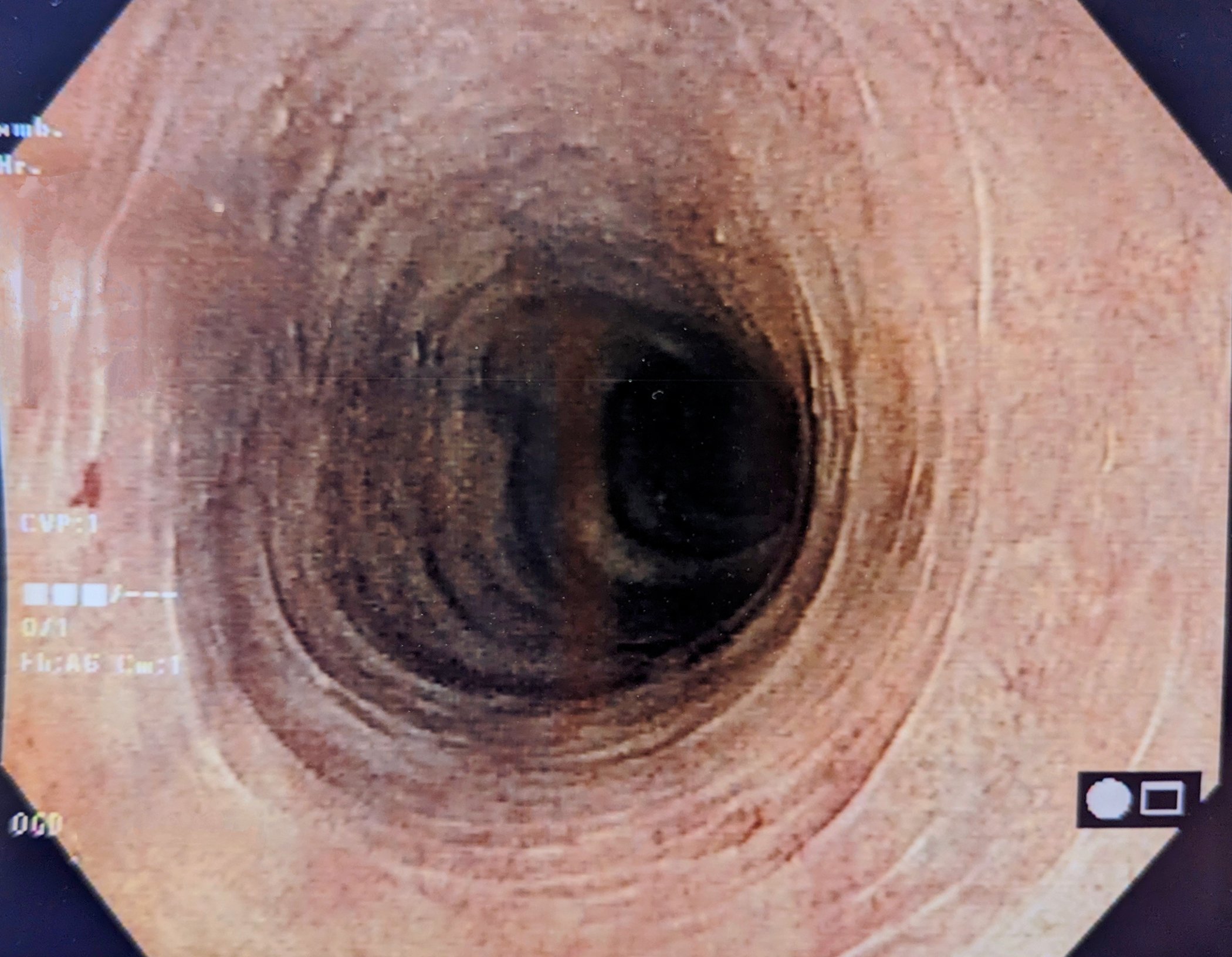
Figure 4 shows an assessment and treatment algorithm for receptive anal intercourse and/or symptoms of proctitis.
Conclusion
Most importantly, if anorectal symptoms are unclear, venereal genesis should also be considered, especially in MSM with a relevant sexual history. In patients with unprotected receptive anal intercourse, asymptomatic proctitis should also be sought by regular smears.
Acknowledgements: Many thanks to Dr. Christoph Riess, who provided all photographs.
Literature:
- Yuhan R, et al: Anorectal disease in HIV-infected patients. Dis Colon Rectum 1998 Nov; 41(11): 1367-1370.
- Spornraft-Ragaller P, Boashie U, Esser S: Sexually transmitted infections of the anorectal region. Dermatologist 2015; 66: 430-438.
- Cohen MS, et al: Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997 Jun 28; 349(9069): 1868-1873.
- Kamarashev J, et al: Lymphogranuloma venereum in Zürich, Switzerland: Chlamydia trachomatis serovar L2 proctitis among men who have sex with men. Swiss Med Wkly 2010; 140: 209-212.
- Kreuter A: Proctology – diseases of the anal region. JDDG 2016; 14(4): 352-375.
- de Vries HJC, et al: 2013 European Guideline on the management of proctitis, proctocolitis and eneteritis caused by sexually transmissible pathogens. Int Journal of STC & AIDS 2014; 25(7): 465-474.
- Schöpfer H, et al: S2k Guideline diagnosis an d thereapy of syphilis- short version. J Dtsch Dermatol Ges 2015; 13: 472-480.
- Fuchs W, Brockmeyer NH: Sexually transmitted infections. J Dtsch Dermatol Ges 2014; 12: 45-63.
- Lanjouw E, et al: 2015 European guideline on the management of Chlamydia trachomatis infections. Int J STD AIDS 2016 Apr; 27(5): 333-348.
- de Vries HJC, et al: 2013 European guideline on the management of lymphogranuloma venereum. JEADV 2015; 29: 1-6.
- Halse TA, Musser KA, Limberger RJ: A multiplexed real-time PCR assay for rapid detection of Chlamydia trachomatis and identification of serovar L-2, the major cause of lymphogranuloma venereum in New York. Mol Cell Probes 2006 Oct; 20(5): 290-297.
DERMATOLOGIE PRAXIS 2016; 26(3): 16-19