There are no validated, sufficiently sensitive and specific laboratory chemistry parameters for the diagnosis of myocarditis or pericarditis. When myocarditis is clinically suspected, elevated cardiac troponins have positive predictive value with respect to biopsy detection. Serologies are of no value in the differential diagnosis of inflammatory heart disease in most cases. Searching for HIV, hepatitis C, tuberculosis, Chagas disease, and Borellia may still be useful depending on the risk constellation. Endomyocardial biopsy is recommended in patients with fulminant, equivocal heart failure or in patients with nonischemic, progressive heart failure despite guideline-compliant therapy.
Inflammatory heart disease in the narrow sense includes myocarditis and pericarditis. Myocarditis is considered a rather rare disease, but most likely underestimated in frequency. Pericarditis, on the other hand, is diagnosed quite frequently. Transitional forms are possible and are defined accordingly as myopericarditis (pericarditis with concomitant myocardial inflammation) and perimyocarditis (myocarditis with concomitant pericardial inflammation) [1]. Clinical presentation is variable, ranging from nonspecific general symptoms to arrhythmias, chest pain, and cardiac failure. In addition to knowledge of epidemiology and clinical expression, the possibilities and limitations of laboratory diagnostics must be known for rational, cost-effective and evidence-based diagnostics.
Epidemiology of pericarditis and myocarditis
The epidemiology of inflammatory heart disease is difficult to grasp. On the one hand, there is no official international consensus on diagnostic criteria, and on the other hand, the clinic is extraordinarily variable, so that the true incidence is most likely underestimated. Indirect evidence for subclinical courses is provided, for example, by prospective studies that documented an asymptomatic increase in cardiac enzymes after smallpox vaccination in up to 3% of subjects [2]. Pericarditis is a disease relevant to everyday life, with an estimated prevalence of 2.7 cases per 100,000 population and approximately 5% of patients presenting to an emergency department with chest pain [3,4]. Myocarditis is much less common, with an estimated incidence of 0.1-1% in the general population. However, the statistical data are difficult to evaluate because of variable diagnostic criteria and the retrospective approach of the studies [5,6]. In Switzerland, an average of 289 inpatient myocarditis cases and 933 inpatient pericarditis cases were registered per year within the last decade (Swiss Federal Statistical Office, inpatient cases for myocarditis 2003-2013).
Causes of inflammatory heart disease
The list of potential causes of inflammatory heart disease is long [7–26]. Basically, infectious, toxic, allergic, immunological and other causes can be differentiated (Fig. 1) . Not infrequently, myo- and pericarditis occur in the context of collagenoses or autoiummune systemic diseases such as celiac disease, systemic sclerosis or lupus erythematosus [7,8]. Central to this is the recognition that myo- and pericarditis have broadly similar etiologies, respectively. may represent pathophysiological and phenotypic variants of the same cause. Pericarditis remains idiopathic in two-thirds of cases. However, if these cases were consistently clarified, possibly up to pericardial biopsy, a viral cause could be found in many cases [9,10].
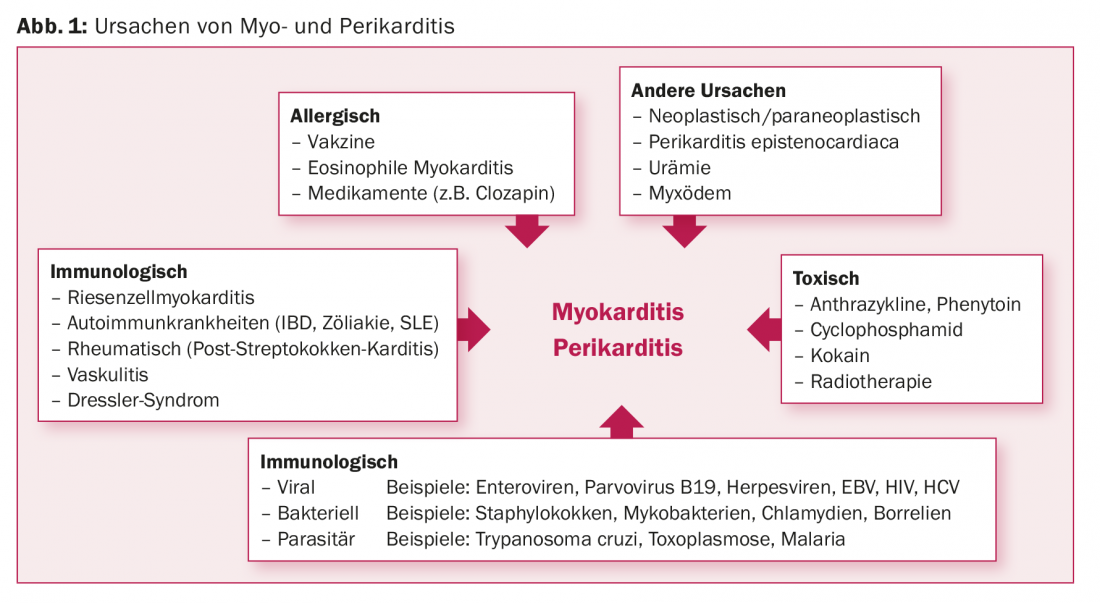
Viruses are considered the most common specific cause in industrialized countries. In myocarditis, interestingly, there is a high geographic variance in the frequency distribution of the causative viruses. Thus, in Germany, polymerase chain reaction (PCR) identified parvoviruses B19 most frequently (19-37%) in endomyocardial biopsies (EMB) from patients with myocarditis [11–18]. In contrast, adenoviruses and enteroviruses were found most frequently in North America (in 20% of 624 patients with biopsy-proven myocarditis) [19]. Finally, in Japan, hepatitis C is frequently identified in EMB [20,21].
HIV infection typically results in a cardiac inflammatory response. Pericardial effusions, usually small, are found in virtually all HIV patients as an expression of subclinical pericarditis. Postmortem analyses showed histologic signs of myocarditis in 67% of HIV patients. The annual incidence of dilated cardiomyopathy (DKM) in non-antiretroviral treated patients is 1.6%. DKM in HIV patients has a poor prognosis [24–26].
Numerous drugs are considered potential triggers for allergic myocarditis. These include tricyclic antidepressants, antibiotics, and antipsychotics [22,23]. However, large prospective studies to assess true incidence and causality are lacking. It is worth mentioning a retrospectively suspected myocarditis incidence of approximately 1% in patients receiving clozapine therapy [23].
Clinic and course of pericarditis
Classically, pericarditis is preceded by nonspecific gastrointestinal or respiratory flu-like symptoms. After a latency of one to three weeks, position-dependent thoracic pain then typically occurs, with radiation to the trapezius margin being almost pathognomonic. The symptoms are more pronounced when the patient is lying down and are often accompanied by severe general symptoms and often subfebrile temperatures. The extent of inflammation, the relevance of the accompanying pericardial effusion, and the frequent occurrence of mostly supraventricular arrhythmias complete the picture of symptoms. Differentially, an acute coronary syndrome must be excluded, in case of doubt also invasively [27]. Often there is no isolated pericarditis, but a more or less pronounced concomitant myocardial inflammation in the sense of myopericarditis. In a retrospective data analysis of 54 patients with myopericarditis, 70% complained of retrosternal pain and 35% of dyspnea at entry. Up to 30% of patients showed signs of heart failure and 57% reported previous influenza infections. The long-term prognosis of pericarditis is good. However, the disease is recurrent in up to 30% [28]. Constrictive pericarditis occurs in <2% and is not clustered even in recurrent courses [29,30].
Clinic and course of myocarditis
Diagnosed myocarditis has a serious prognosis, with an estimated 1-year mortality of 15-20% and a 4-year mortality rate above 50% [31–35]. Patients with the rare form of giant cell myocarditis have a particularly high 5-year mortality rate of over 80% [36]. Liebermann et al. distinguish patients with fulminant myocarditis, i.e. rapidly progressive over two weeks, and rapid heart failure, but paradoxically good long-term prognosis (93% transplant-free survival after five years), from patients with acute, slowly progressive myocarditis with a worse long-term prognosis [31,37]. The good long-term prognosis of fulminant myocarditis warrants aggressive, intensive medical management including the use of assist devices. Overall, the spontaneous recovery rate of clinically symptomatic myocarditis under drug therapy for heart failure and physical rest is estimated to be more than 50% [38,39].
Epidemiological and experimental data support an association between myocarditis and dilated cardiomyopathy (DKM). This is supported, among others, by the detection of specific inflammatory cells such as T cells or macrophages and the increased expression of inflammatory markers and adhesion molecules in the EMB of some patients with DKM [40]. Prospective long-term studies in patients with histologically confirmed myocarditis estimate the proportion of those who develop DKM to be as high as 52%, depending on diagnostic criteria and patient selection [38]. Finally, myocarditis also plays a role in sudden cardiac death. Histological myocarditis criteria are met in 8.6-12% of autopsy-examined hearts from patients with sudden cardiac death [41,42]. Perimortem inflammatory reactions must be differentiated from myocarditis proper.
General value of laboratory diagnostics in inflammatory heart disease.
Diagnosis of myocarditis or pericarditis is made clinically, by excluding other, usually common, differential diagnoses, and by using more or less specific imaging techniques. In the latter, cardiac magnetic resonance imaging currently provides the most comprehensive information. However, the gold standard for myocarditis diagnosis is EMB. Laboratory chemical analyses provide important information on etiology, differential diagnosis, and prognosis. (Table 1) . However, specific diagnostic markers for myo- or pericarditis do not exist. Available laboratory analyses must be targeted in the clinical context, taking into account pretest probability, which is increased by clinical, epidemiologic considerations, and exclusion of common heart disease.
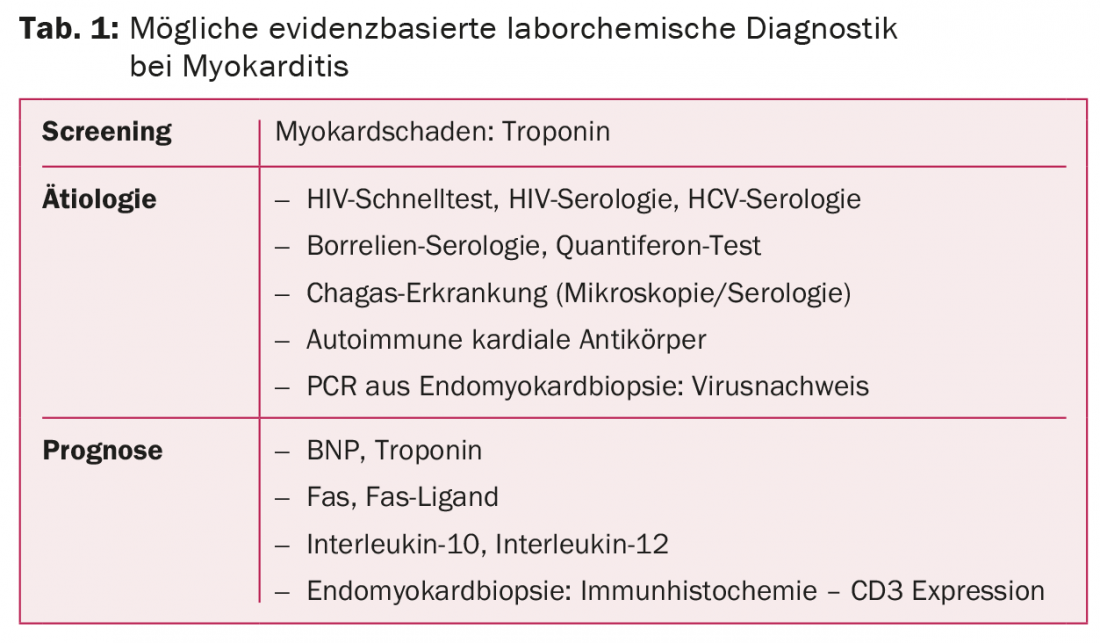
In patients with pericarditis, inflammatory parameters such as leukocytes and C-reactive protein (CRP) are usually elevated. However, this does not apply to myocarditis. Here, normal values by no means exclude myopericardial inflammation [43–45]. Cardiac enzymes that can be determined in serum are cardiac troponins and creatinine kinases (CK). The latter have no place in the diagnosis of inflammatory heart disease because of their low sensitivity and specificity [43,46,47]. In contrast, cardiac troponins are very often initially elevated (30-80%) in both myo- and pericarditis. In patients with suspected myocarditis, high troponin levels increase the pretest probability for biopsy confirmation. On the other hand, the negative predictive value of cardiac troponin T (cTnT) of less than 60% is not useful to exclude myocarditis [43,46,48,49]. Interestingly, patients with biopsy-proven myocarditis but negative troponin are found to have significantly longer symptom duration to diagnosis than patients with elevated troponin [46]. However, troponin levels that are elevated at diagnosis have no predictive value for adverse outcome or recurrence [9,50–52]. Although they are organ-specific, troponins and troponin profiles are only moderately suitable for the differential diagnosis of a defined heart disease.
Pericarditis: clinical value of laboratory chemistry.
Pericarditis is 85-90% idiopathic in developed countries. Case series demonstrate that the consistent search for a specific etiology in pericarditis initially judged to be idiopathic offers no prognostic advantages [53–55]. Thus, excessive etiologic investigations are of little value in immunocompetent patients. This is especially true for “Rheumaserologies” resp. the search for antinuclear antibodies and rheumatoid factors. Such investigations are only appropriate if there is further clinical or anamnestic evidence of collagenosis, vasculitis or other systemic autoimmune disease [53].
In contrast, in sub-Saharan Africa and especially with concurrent HIV infection, tuberculosis is responsible for 70-80% of pericarditis cases. This fact should be taken into account in patients with HIV infection, a corresponding history of residence or immunosuppression with an appropriate clarification. Blood cultures should be obtained in patients with pericarditis and a septic state [56]. Risk indicators for a serious course and a non-idiopathic etiology include high fever, subacute prolonged symptoms, large pericardial effusions (>20 mm), pericardial tamponade, and lack of response to anti-inflammatory drugs. These patients should be evaluated in more detail [9]. Pericardiocentesis with histochemical and cultural analysis is recommended only in patients with tamponade, large effusion, and a risk constellation for neoplasia or tuberculosis in previously nonconclusive investigations [57]. Persistently elevated CRP levels under adequate anti-inflammatory therapy are considered the only prognostic marker for recurrent pericarditis [45]. Whether prolonged anti-inflammatory therapy for persistently elevated CRP can prevent recurrences in the long term has not been investigated.
Myocarditis: clinical significance of laboratory chemistry.
In appropriate clinical settings, elevated troponin levels are suggestive of myocarditis after exclusion of other etiologies (coronary artery disease, tachyarrhythmias, hypertensive danger, chronic heart failure) [50]. Recently, it has been shown that elevated cTnT levels are not only associated with a greater likelihood of biopsy-proven myocarditis but are significantly higher in acute myocarditis than in chronic courses [50]. The newer laboratory chemical parameters copeptin and “mid-regional pro-adrenomodullin” also investigated in this study showed no diagnostic or prognostic benefit. For this, markedly elevated blood concentrations of brain natriuretic peptide (BNP) above 4245 pg/ml were associated with significantly increased mortality within one year [50].
The cellular surface proteins sFas and sFas ligand were identified in the late 1990s as apoptosis markers in heart failure and myocarditis. Their serum concentration correlates with the symptomatic phenotype or NYHA stage of the disease [58–60]. A Japanese study investigated the diagnostic and prognostic value of these serum parameters in myocarditis. sFas and sFas ligand were significantly higher in patients with myocarditis versus healthy volunteers or patients with status post myocardial infarction. The level of serum concentrations of the two biomarkers was predictive of clinical outcome (“fatal” versus “recovery”) in a retrospective study of patients with fulminant myocarditis [61]. Interleukin-10 and interleukin-12 have been identified as additional prognostic serum markers in several small studies [62,63]. However, prospective studies on the diagnostic value of sFas, sFas ligand, interleukin-10, and interleukin-12 in EMB-proven myocarditis and a cost-stratified benefit analysis of these serum markers do not exist.
Systematic and broad serologic workups, especially viral serologies, have no place in the diagnosis of myocarditis. Kandolf et al. demonstrated strikingly low sensitivity and specificity of viral serologies compared with molecular biological virus identification in cardiac biopsy based on a prospective study of 124 patients with suspected myocarditis. Only in 9% of patients with viral detection in cardiac biopsy could the virus also be confirmed serologically [44]. Exceptions are at-risk populations with history or clinical evidence of very specific viral or bacterial infections. These include infections with HIV or hepatitis B/C[24–26]. With increasing incidence of Lyme borrelliosis with a carditis prevalence of 0.3-4%, Lyme serology in endemic areas is useful [64]. Due to increasing migration patterns, Chagas disease, which is primarily endemic to South America and caused by the parasite Trypanosomi cruzii, is also gaining importance. Studies estimate the current prevalence in Europe at 80,000-100,000 individuals, which is approximately 4% of immigrant South Americans [65,66]. Chagas disease leads to DKM via persistent myocardial inflammation [67]. In individuals with recent onset of heart failure and/or arrhythmias and appropriate migration or travel history, Chagas disease must be actively sought by blood smear (microscopy) and serology. This is even more so because timely treatment limits the progression of cardiac complications [68].
Value of endomyocardial biopsy in myocarditis.
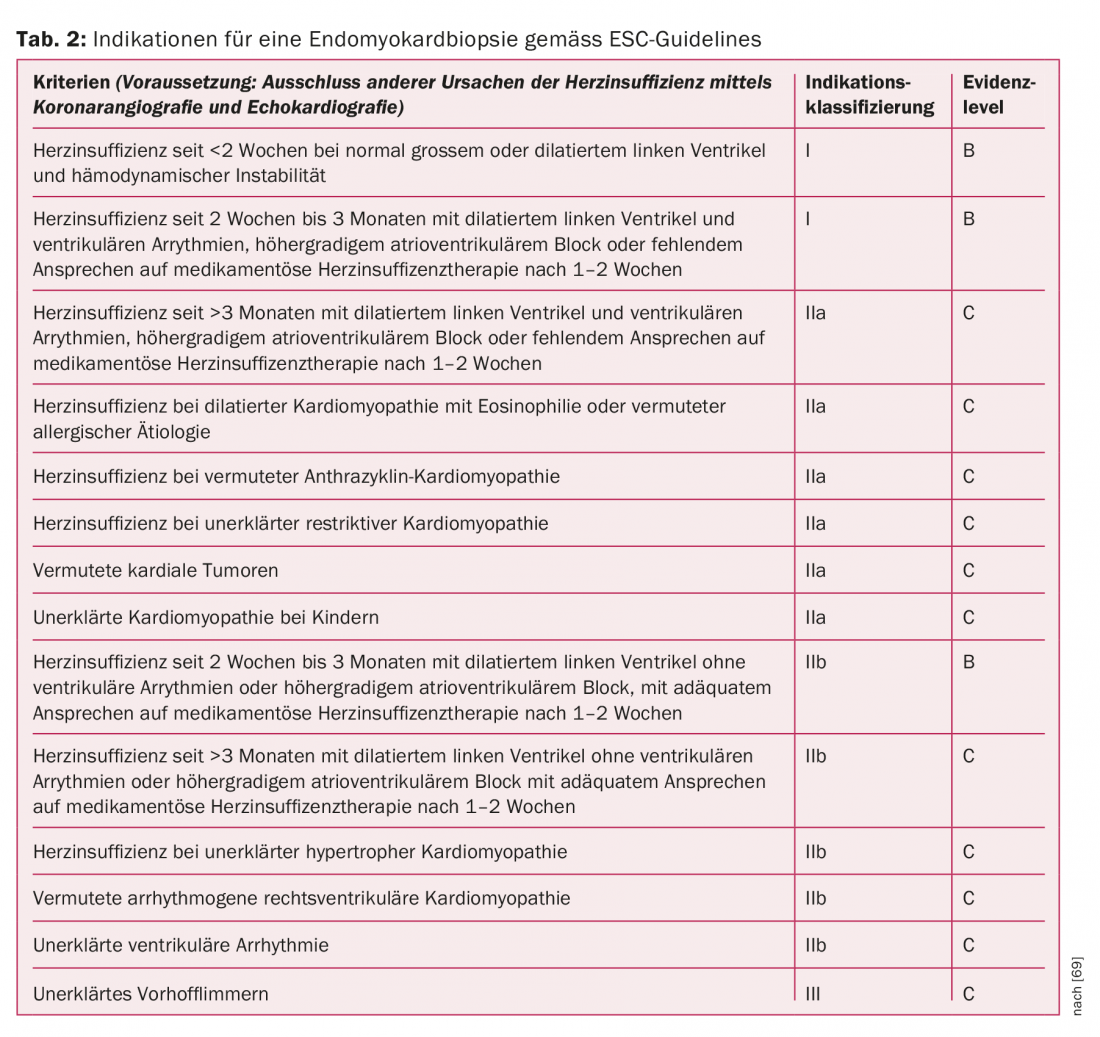
Immunohistological and molecular biological analysis of EMB has diagnostic, prognostic, and therapeutic implications. In the currently valid 2007 recommendations of the European Society of Cardiology (ESC), the indication for EMB is based on patient characteristics, duration of heart failure therapy, complications, and other criteria (Table 2) [69]. With a complication rate of 6% (>3% transient arrhythmias and accidental arterial punctures), the examination is considered relatively safe with appropriate experience of the interventionalist [70]. Although EMB is the diagnostic gold standard, its sensitivity is not without controversy. The limited sensitivity is based on the one hand on the often focal inflammatory infiltration pattern and the associated “sampling error”. On the other hand, data from animal studies suggest that the composition of inflammatory infiltrates can change rapidly during the course of the disease. This is also the reason why for myocarditis diagnosis the historical, purely histologically defined Dallas criteria of 1998 have been replaced by molecular biological and immunohistochemical criteria with higher sensitivity and specificity [71–75].
Bioptic evidence of myocardial inflammation is considered a negative predictor of subsequent outcome and graft-free survival. However, this is not true for molecular detection of a cardiotropic viral pathogen, which is successful in approximately 40% of EMB [19,76]. Immunohistochemical detection of inflammation with concomitant viral detection in EMB is likely to be the basis for potential antiviral therapy in the future. In the absence of viral detection but confirmed inflammation, an immunosuppressive therapy trial is justifiable. The prognostic significance of virus persistence in serially obtained EMBs cannot be conclusively assessed at present [77,78]. In addition to immunohistological inflammation detection and molecular pathogen detection, EMB also allows identification of specific myocarditis subgroups with potentially specific therapeutic implications (sarcoidosis, giant cell myocarditis, myocarditis in hypersensitivity syndrome) [10].
Cardiac-specific autoimmune antibodies in myocarditis.
Cardiac-specific autoimmune antibodies (AHA) can be determined by immunofluorescence or ELISA in up to 60% of patients with clinically diagnosed myocarditis and in 35% of patients with biopsy-confirmed myocarditis, whereas they are undetectable in cardiac healthy subjects. Accordingly, an autoimmune genesis is postulated for a subset of myocarditis with negative viral detection and persistent inflammation in the EMB and detection of AHA in the serum [79–81]. For example, a recently published prospective study by Caforio et al. with 174 patients with biopsy-confirmed myocarditis showed evidence of AHA in 48% of subjects with concurrent negative biopsy virus detection [39]. Interestingly, AHA can also be detected in a proportion of patients with DKM and, in some cases, in the serum of their asymptomatic relatives. On the one hand, these data support the concept that DKM represents the late sequelae of myocarditis; on the other hand, they argue for a potentially hereditary contributory autoimmune genesis of DKM [82,83].
Therapeutic concepts
Therapy for idiopathic pericarditis consists mainly of nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine [57]. The latter reduces the risk of recurrence and accelerates the clinical course of healing [84]. Because of the increased likelihood of recurrence with persistently elevated CRP, dose reduction of anti-inflammatory therapy should only be performed under laboratory control [45].
In patients with high-grade suspicion of myocarditis, inpatient workup is essential because the short-term course cannot be predicted and important therapeutic consequences may arise in the short term during diagnostic workup [85]. Hemodynamically unstable patients need aggressive circulatory support early because, after survival of the fulminant phase, the long-term prognosis is favorable [31]. In addition to a ban on sports, all patients with myocarditis and impaired left ventricular function qualify for heart failure drug therapy according to the usual regimen [86]. NSAIDs and colchicine have no therapeutic value in myocarditis [10]. Because no larger multicenter studies have investigated immunomodulatory, immunosuppressive, or antiviral therapies in specific subgroups of myocarditis, the relevant recommendations are based on studies with small patient numbers and expert consensus [10]. The rationale is that a differentiated, structered, and rational diagnosis will allow the classification of myocarditis into distinct subgroups with appropriate therapeutic options (Fig. 2).
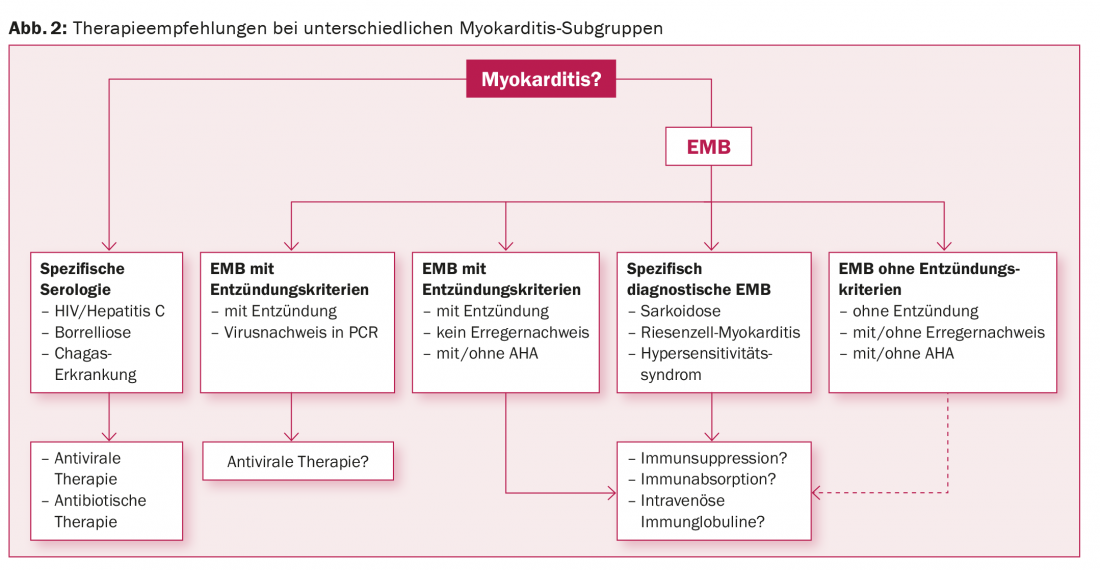
In patients with HIV, Chagas, borrelliosis or tuberculosis, the focus is on therapy for these diseases. If inflammation is detected with concomitant viral detection in the EMB, antiviral therapy options are based on the viral type. In the absence of viral detection, immunosuppressive therapy is basically an option; the potentially beneficial effect in bioptically confirmed, virus-negative myocarditis has already been demonstrated, at least with regard to echocardiographically recorded functional parameters [87]. For the future, we hope for international, multicenter, prospective randomized controlled trials with large patient numbers.
Literature list at the publisher
CARDIOVASC 2015; 14(4): 22-29