Epilepsy is considered one of the most common chronic neurological conditions and severely limits the quality of life of those affected. Despite a wide range of options, current therapeutic options often reach their limits. In order to increase disease control, an innovative stimulation procedure has been introduced and is currently in the clinical testing phase.
Despite the introduction of new pharmacological treatment options, up to 60% of epilepsy patients do not respond or do not respond adequately to currently available antiepileptic drugs, depending on their severity. Even with dual or triple administration, the desired improvements cannot be achieved in about one in three patients, reported Prof. Andreas Schulze-Bonhage, Freiburg, MD. Quality of life suffers as a result, because leisure activities, for example, must also be adapted to the disease, since the cognitive impairments increase the risk of injury, among other things. “Also, some professions cannot be practiced, and because of possible stigma, social roles in particular, such as taking a lecture, are avoided,” the expert said.
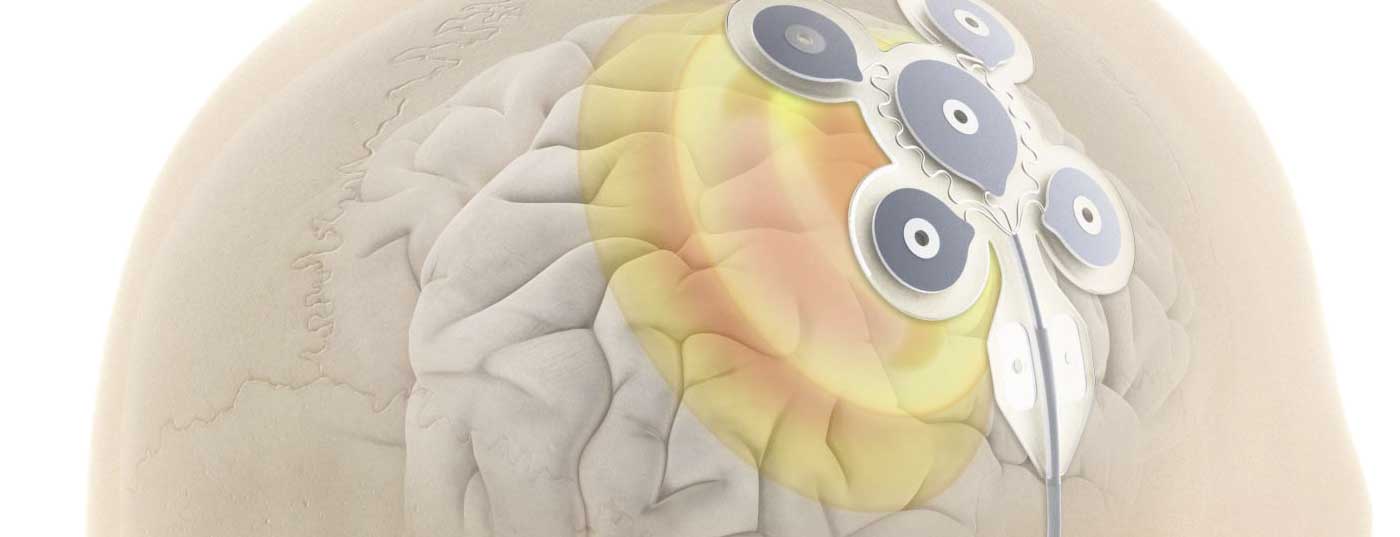
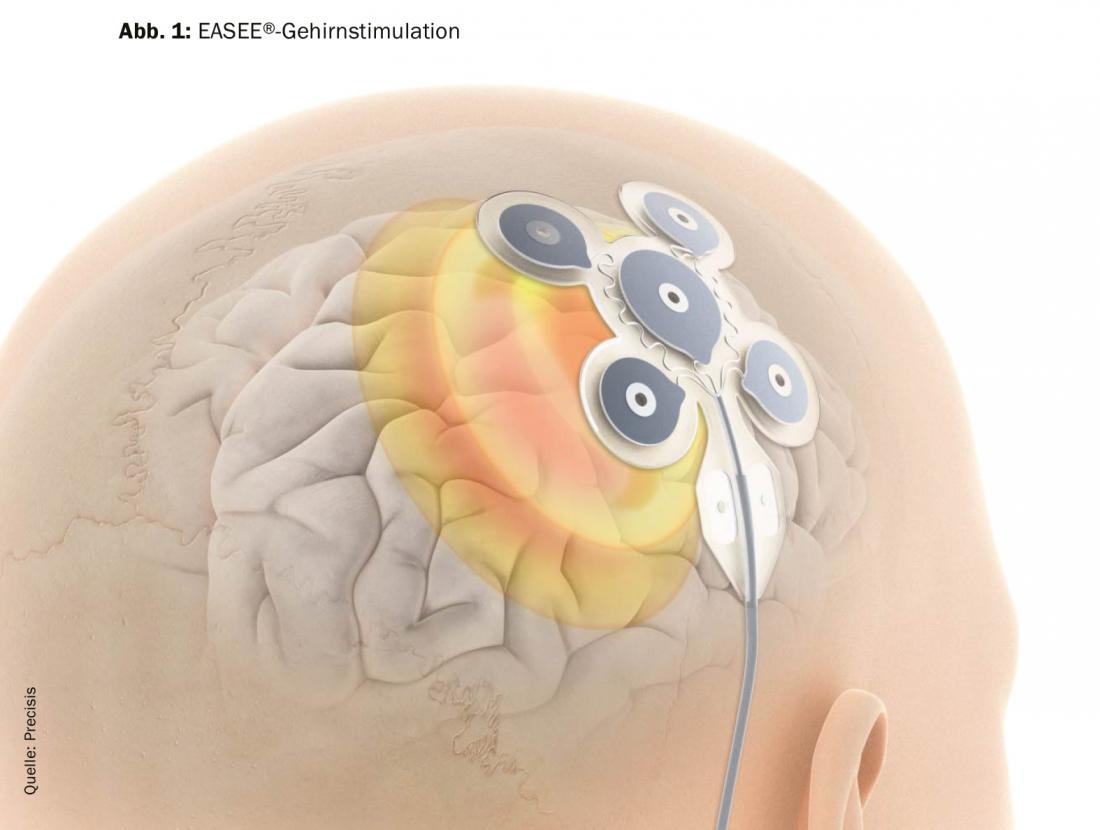
One possibility of therapy is epilepsy surgery, which can achieve very good results with seizure freedom of up to 70%, but is only open to a very small group of patients. Therefore, EASEE®, a minimally invasive, fully reversible and transcranial stimulation procedure, has now been developed (Fig. 1). A thin silicone mat with integrated platelet electrodes is placed over the epileptic center with anatomical precision and provides optimal stimulation by combining two patterns that have already been clinically successful. Through AC stimulation and ULFA stimulation, a pulse is delivered every two minutes for 500 ms and an additional long pulse is generated once a day for 20 minutes.
In clinical testing
Currently, the EASEE® II study is ongoing to demonstrate the safety of the EASEE® system in patients with pharmacoresistant focal epilepsy. In addition, the effects on seizure frequency, seizure severity, epileptic activity on EEG, quality of life, and mood and cognition will be recorded and evaluated. The first implantations have been performed and the first positive effects have already been documented. “Especially patients with focal epilepsy and an inadequate response to antiepileptic drugs, for whom surgery is not an option or who refuse it, could benefit from the new method,” Schulze-Bonhage is convinced.
Source: Precisis
InFo NEUROLOGY & PSYCHIATRY 2019; 17(6): 40.