Small changes made in the 2022 BCLC update may be of great impact in the local therapy of stage 0 to C HCC patients. Combination therapies and other interdisciplinary measures show curative improvements. Which patients are suitable for which types of therapy at which stage of disease will be explained based on the possibilities, limitations, and future prospects of locoregional therapies in HCC.
A recent extension of the representation of the BCLC algorithm in the 2022 BCLC standard [1] has given rise to discussion. Although the types and pathways of therapy for hepatocellular carcinoma (HCC) outlined therein were only supplemented by minor differences. However, it is precisely these “side steps” that represent interesting and good opportunities, explained Professor Dr. Roman Klöckner from the Clinic and Polyclinic for Diagnostic and Interventional Radiology at the University Medical Center Mainz (D) [2].
For the very early (0) and early (A) stage of HCC, the therapeutic options so far have been ablation, resection, and possibly already transplantation. For example, the right choice for an individual patient depends on center expertise, interventional radiology capabilities, transplant option, and the experience of the liver surgeon. A particular challenge, however, is posed by the patients themselves, whose clinical pictures are known to be highly complex and with very heterogeneous tumor processes. The very fact that one always has to deal with two simultaneous clinical pictures – with liver cirrhosis on the one hand and HCC on the other. Because of the many different tumor pictures and a range of interventional options, interdisciplinary discussion of each individual patient in the tumor board is therefore essential, says Prof. Klöckner. These patients would have to be discussed intensively, not just once, but before each new measure or cycle.
A possible curative local therapy for very early and early hepatocellular carcinoma is to “cook” it minimally invasively by ablation and use of micro- or radio-waves. Professor Klöckner referred to the so-called “No-Touch Technique” as an example, which does not puncture directly in the tumor but immediately adjacent to it. This approach enjoys very good results and also has three advantages, as the expert explained:
- Reduced risk of tumor cell engraftment
- Risk of tumor rupture in exophytic foci practically zero
- Complication of biliopleural fistula under control
Although the recurrence rates were higher than in surgery across all studies, they did not translate into a worse survival rate, so that ablation could be considered equivalent to surgery.
Powerful combo: ablation + TACE
The combination with trans-arterial percutaneous chemoembolization (TACE) makes it possible to ablate tumors up to a cut-off of 7 cm. This is because blood flow comes to a standstill in the well-embolized tumor. This eliminates the tumor’s own cooling, resulting in a more homogeneous heat propagation and thus somewhat enlarged ablation zones. If necessary, ablate with two needles and from several positions or overlapping.
Even in this stage, which until recently was therapeutically more or less dominated by radiologists, there are changes. Significantly improved systems therapy already offers a complete alternative to radiology in some patients, and again, interdisciplinary discussion leads the way in placing the patient on the treatment pathway. Today, according to Prof. Klöckner, one should no longer make the mistake of winding up the patient once, who then ends up on a “TACE endless track.” The challenges, particularly with BCLC B, are more complex than they might appear on an S3 flow chart, he said, and many of them are not mapped in the guideline. These include the varying tumor burden and liver function, the growth pattern and location of the tumor, the vascular supply, etc. Not all patients respond equally well to TACE and sometimes benefit more, sometimes less from this therapy.
Many factors determine the pros and cons of locoregional therapy (overview 1), including, for example, vascular problems: if a patient has a biliary stent, this argues against TACE and in favor of systems therapy or radioembolization. The latter does not lead to direct necrosis and therefore does not lead to a risk of infection, as can happen with TACE, but slowly decomposes the tumor.

Small side steps – big effect
The classification into pro and contra TACE shows the dimension of the small side-steps into the types of therapy mentioned at the beginning: those cases classified as “contra TACE” and which are classified in the intermediate stage in the guideline would have been embolized earlier. Today, they are in any case primarily treated with systemic therapy and can be successfully followed up with additional locoregional therapy if the response is good.
It is possible to destroy a high tumor burden with TACE. However, because TACE response has been shown to be linked to tumor size, we know that such tumors do not respond as well. There is also a risk of severe post-embolization syndrome with high tumor burden.
Macrovascular invasion is often overlooked by the radiologist, and yet it affects one-third of patients during the course of the disease. Even those formally classified as BCLC C, which would be clear “systems therapy candidates,” may now be treatable locoregionally. Some data, especially from Japan, show curative approach even for surgical intervention at this stage for small infiltrations. Due to the very poor data situation, the last word has not yet been spoken. Prof. Klöckner sees TACE critically here, if, then he would still treat with SIRT.
SIRT: only suitable for a few – but then really good
Due to the poor data situation caused by the failed SARAH and SIRveNIB trials, SIRT was more or less written off. In the advanced stage, with few exceptions, it is no longer an issue, especially since it is also expensive and needs good justification. Nevertheless, there are also small niches in which SIRT provides valuable results in Prof. Klöckner’s view.
In patients whom one does not want to operate because of hypertension or whom one also does not want to ablate because of the proximity of the tumor to the bile duct or to the vessel, one could have success with superselective SIRT – at least at the segmental level or even more selectively. A correspondingly individualized dosimetry is required. With min. 210 Gray tumoricidal dose into the four-digit range in some cases, the typically triangular-shaped segment is treated. In this case, SIRT is curative, although not overnight.
One final change involves the inclusion of the Treatment Stage Migration Concept in the BCLC update. This is important, he said, because it allows therapists to change the normally scheduled therapy on the guideline pathway with good reason. For example, to follow up a patient who has responded excellently to system therapy with locoregional therapy. Prof. Klöckner once again emphasized the importance of good exchange between all disciplines in order to do justice to these complex patients.
TACE/SIRT plus immunotherapy – new fire for the system?
The combination of TACE and TKI has been shown to be negative in several studies, ultimately the toxicity was too high and therefore compliance was poor. This contrasts with the combination of TACE/SIRT plus immunotherapy, which has a more favorable side effect profile and is therefore much easier to combine (Fig. 1) [3].
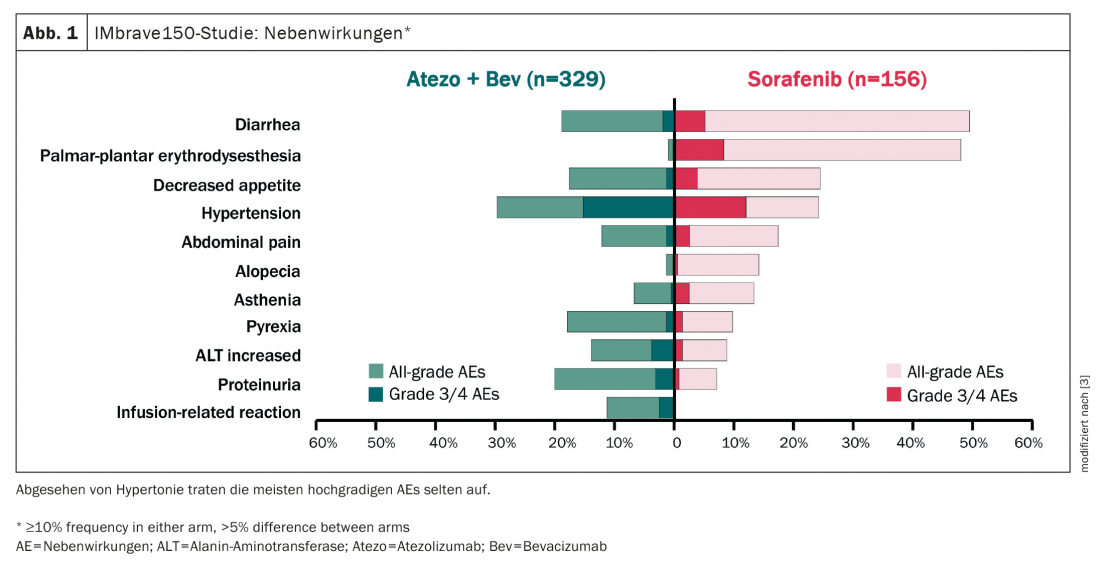
Such a combination also holds out the hope of fueling immune system stimulation with combination therapy. Response to immunotherapy alone was still poor at 27%, he said. However, together with TACE/SIRT, it is hoped to achieve higher response rates via the pathway of tumor destruction, release of TAAs, stimulation of the immune system, and hopefully a stronger response of immunotherapeutics against HCC. In the ideal therapeutic case, not only an additive but even a synergetic effect occurs.
Congress: GI-Oncology 2022
Literature:
- Reig M, Forner A, Rimola J, et al: BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022; 76(3): 681-693; doi: 10.1016/j.jhep.2021.11.018.
- GI-Oncology 2022 – 18th Interdisciplinary Update; face-to-face event on 11.6.2022 in Wiesbaden (D).
- Galle PR, Finn RS, Qin S, et al: ASCO GI 2020; Abstr 476; doi: 10.1200/JCO.2020.38.4_suppl.476.
InFo ONCOLOGY & HEMATOLOGY 2022; 10(4): 28-29.