Iron deficiency is a common comorbidity in chronic heart failure and is associated with increased morbidity and mortality independent of the presence of anemia. In chronic heart failure, annual anemia screening including. Iron status (serum ferritin, transferrin saturation) and CRP are performed. The diagnostic criteria of iron deficiency according to the FAIR-HF study are: serum ferritin <100 μg/l (absolute iron deficiency), serum ferritin 100-299 μg/l and transferrin saturation <20% (functional iron deficiency). For symptom improvement, parenteral iron substitution, preferably using iron carboxymaltose, should be considered in symptomatic systolic heart failure.
Chronic heart failure is a common disease, affects many organ systems, and still has a poor prognosis despite well-established treatment guidelines. This is one of the reasons why research on modifiable risk factors has increased in importance in recent years. Anemia and iron deficiency as frequent cofactors play a prognostically important role. The prevalence of iron deficiency in patients with chronic heart failure is approximately 50% [1]. Hospitalized patients with NYHA IV heart failure and anemia showed iron deficiency in as many as 73% according to bone marrow biopsies [2]. The presence of anemia or iron deficiency is also an independent and strong predictor of poor prognosis [3]. In the largest FAIR-HF (“Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency”) study to date, correction of iron deficiency by intravenous iron supplementation had no effect on mortality and morbidity but significantly improved exercise capacity and heart failure symptoms in patients with systolic heart failure, even in the presence of iron deficiency without anemia [4]. Other approaches to the treatment of anemia, such as erythropoietin derivatives, have also been studied several times. Because of their controversial efficacy and long-term cardiovascular side effects, they are not mentioned in the ESC (The European Society of Cardiology) guidelines for chronic heart failure therapy [5].
Pathophysiology
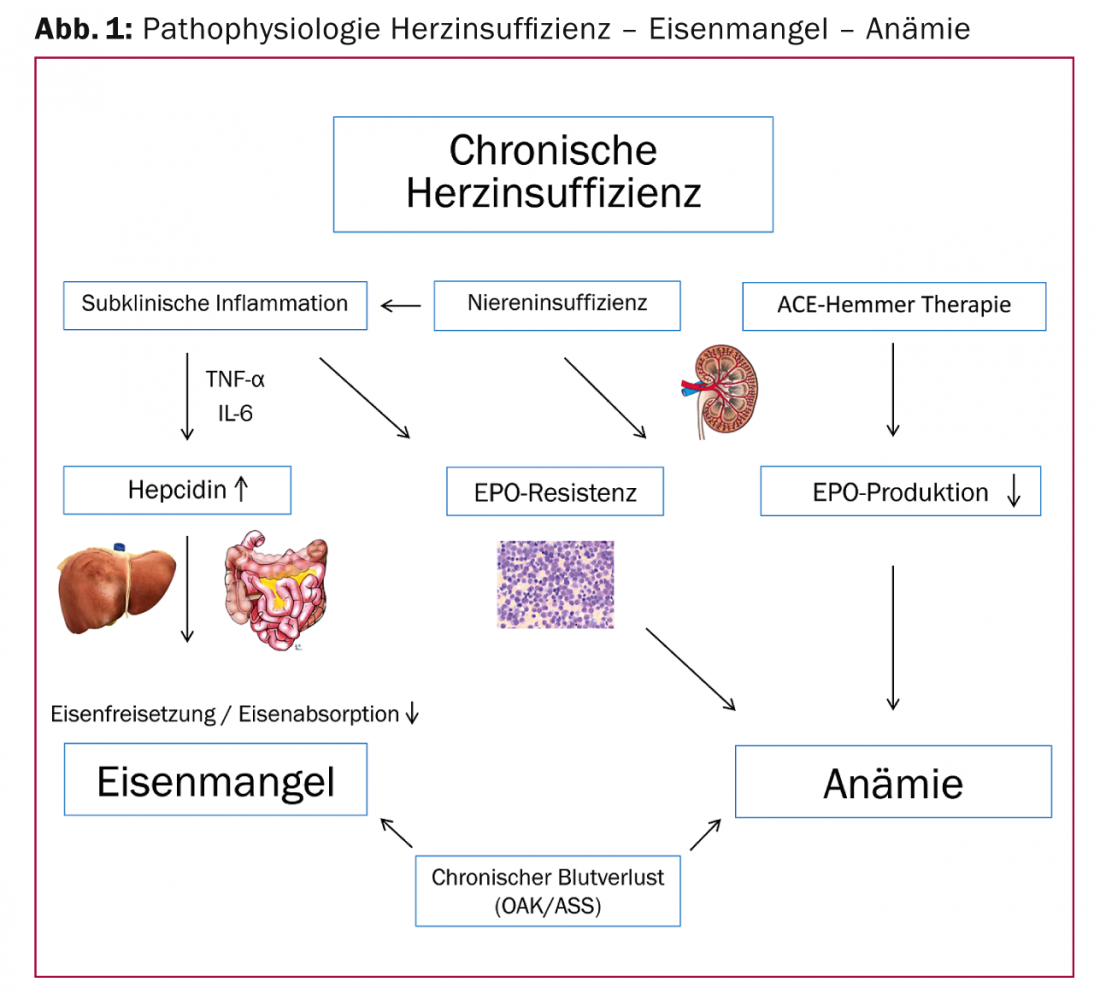
The underlying mechanisms of anemia or iron deficiency are multifactorial and largely analogous to those of other chronic systemic diseases such as rheumatoid arthritis or Crohn’s disease. By far the most common forms of anemia in heart failure are chronic inflammatory anemia (60%), followed by iron deficiency anemia (21%) [3]. Often this is a “functional” iron deficiency, where iron stores are adequately filled but release and intestinal iron absorption, and thus bioavailability, are inhibited. In addition to erythropoiesis, iron also has important functions in metabolic processes in cardiac and skeletal muscle (respiratory chain enzyme), which can influence performance.
Chronic inflammation
Heart failure is associated with a chronic inflammatory process that is usually subclinical. This leads to the release of pro-inflammatory cytokines (IL-6) and tumor necrosis factor (TNF), which decrease EPO production and sensitivity of the bone marrow to EPO. In addition, IL-6 promotes hepatic synthesis and release of the acute-phase protein hepcidin. This in turn inhibits ferroportin, so that intestinal iron absorption and mobilization from stores are inhibited.
Other important cofactors that may cause anemia or iron deficiency include decreased erythropoietin production in chronic renal failure, vitamin B12 or folic acid deficiency due to malnutrition, and occult gastrointestinal blood loss with oral anticoagulants. The use of ACE inhibitors and angiotensin receptor blockers may also favor the development of anemia by inhibiting EPO synthesis [6] (Fig. 1).
Iron deficiency diagnosis
Every patient with symptomatic heart failure NYHA II should have anemia screening including. Iron status (blood count, plasma ferritin, transferrin saturation, soluble transferrin receptor) should be done. This is also in accordance with the ESC guidelines 2012 [7]. In the presence of anemia (Hb <12 g/dL in women, Hb <13 g/dL in men), routine testing of the most common causes of anemia should be performed. Serum ferritin, as an acute-phase protein, may be elevated with inflammatory activity, so a normal serum ferritin does not rule out iron deficiency. Therefore, a CRP should always also be determined to estimate the inflammatory activity and diagnostic value of the serum ferritin.
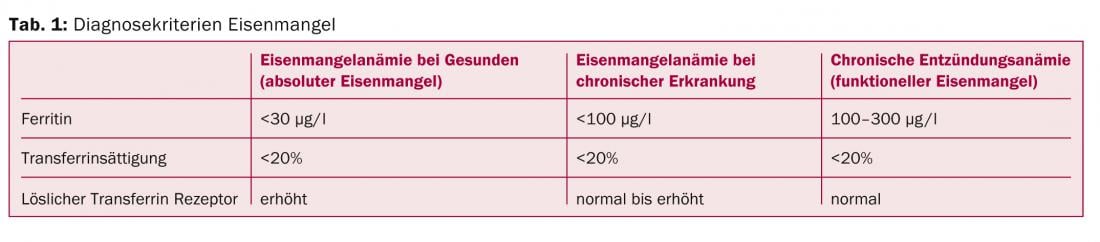
Regarding serum ferritin, reference values in healthy individuals are approximately >20 μg/l in women and >30 μg/l in men. In chronic diseases, however, iron deficiency may already be present at a ferritin of <100 μg/l.
Differentiation between absolute and functional iron deficiency in chronic inflammatory anemia can be made on the basis of serum ferritin and transferrin saturation (Table 1).
In the case of absolute iron deficiency anemia, gastrointestinal blood loss should also be excluded.
Calculation of the iron deficit and demand
The Ganzoni formula (Fig. 2), which has been known since 1970, is still the most commonly used method for calculating the iron deficit [8]. Based on body weight and target hemoglobin, iron requirements can be estimated.

According to the manufacturer’s recommendation of iron carboxymaltose (Ferinject®), a target Hb of 13 g/dL and a reserve iron of 15 mg/kg should be used for body weights below 35 kg. For body weight above 35 kg, a target Hb of 15 g/dL and a constant reserve iron of 500 mg is recommended. As a rule of thumb, an iron deficit of about 1000 mg of iron can be assumed for the majority of patients.
Therapy of iron deficiency – How to substitute?
Oral substitution: Previous experience has shown that oral iron substitution, especially in patients with chronic heart failure, has had little success due to pathophysiologic intestinal wall changes, unfavorable side effect profile, and duration of therapy. In patients with chronic inflammatory anemia, orally administered iron was poorly absorbed and could inadequately fill iron stores as measured by serum ferritin [9].
Intravenous iron substitution: parenteral iron application bypasses impaired enteral absorption and also hepcidin-mediated inhibition of iron release. In the 2012 ESC guidelines, parenteral iron substitution using iron carboxymaltose used in the FAIR-HF study is also mentioned for the first time as a therapeutic option for iron deficiency or iron deficiency anemia in heart failure [7].
The FAIR-HF study was published in 2009 and was the first larger prospective double-blind, placebo-controlled study to investigate the treatment of iron deficiency in heart failure using i.v. ferric carboxymaltose [4]. A total of 459 patients with systolic heart failure (NYHA II [LVEF <40%] and NYHA III [LVEF <45%]) and iron deficiency (serum ferritin <100 μg/l or serum ferritin of 100-299 μg/l and transferrin saturation <20%) were randomized 2:1 (therapy vs. placebo). Both patients with and without anemia were included for a hemoglobin range of 9.5-13.3 g/dL.
Using the Ganzoni formula described previously, the iron deficit was calculated and 200 mg iron carboxymaltose was administered i.v. weekly until the calculated iron deficit was replenished. Subsequently, a maintenance dose of 200 mg i.v. was given every four weeks until week 24 of the study. After 24 weeks, there was a significant improvement in the primary endpoints of subjective health status (“self-reported patient global assessment”) and NYHA classification.
The factors predefined as secondary endpoints, physical performance (distance in the 6-minute walk test) and subjective quality of life, were also significantly improved in the ferric carboxymaltose group after only four weeks. Subgroup analyses showed similar benefit in patients with or without underlying anemia. There were no differences in all-cause mortality, morbidity, and hospitalizations between the study groups.
These results also confirmed the results of previously published, smaller studies, all of which demonstrated favorable therapeutic effects of i.v. iron supplementation as measured by exercise capacity, NYHA classification, and quality of life in patients with chronic heart failure and anemia or iron deficiency [10–12].
Erythropoiesis-stimulating agents (ESAs): the use of darbepoietin in patients with systolic heart failure and anemia was investigated, among others, in the RED-HF(“Reduction of Events by Darbepoetin Alfa in Heart Failure”) study published in 2013 [5]. A significant survival benefit with darbepoietin was not shown, but significantly more thromboembolic complications were found in the darbepoietin group. Thus, anemia treatment with ESAs is generally not recommended in patients with chronic heart failure unless indicated by nephrology.
Conclusions and therapy recommendations for practice
Iron deficiency and anemia are prognostically important cofactors and show a high prevalence in patients with chronic heart failure. They have a significant impact on quality of life and performance. An annual routine laboratory screening with blood count and iron status incl. Serum ferritin and transferrin saturation should be done in every patient with symptomatic heart failure. If unexplained iron deficiency is present, differential diagnostic anemia workup, especially endoscopic hemorrhage search, is indicated.
To improve patients’ symptomatology and performance, we recommend considering parenteral iron supplementation, analogous to the inclusion criteria of the FAIR-HF study, in patients with a serum ferritin <100 μg/l or a serum ferritin between 100 and 299 μg/l and a transferrin saturation <20%. Based on current data and the pathophysiologically poor iron availability in heart failure, low-dose replacement therapy with 200 mg i.v. once a week is recommended until the calculated iron deficit is reached (see Ganzoni formula).
Whether administration of higher single doses (e.g., 500 mg, 1000 mg) is equally safe and effective in heart failure is currently being investigated in clinical trials. In terms of the evidence to date regarding symptom improvement and the more favorable side effect profile, we believe that dextran-free ferric carboxymaltose is preferable to ferrous sucrose.
Further adequately powered, placebo-controlled trials are needed to assess the effect of iron supplementation in patients with heart failure with respect to the hard end points of mortality, hospitalizations, and long-term effects. At the present time, the question also remains unresolved whether intravenous iron supplementation provides the same benefit in patients with heart failure with preserved ejection fraction. In this regard, the FAIR-HFpEF study is being planned.
Lukas Meier, MD
Literature:
- Klip IT, et al: Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4): 575-582.e3.
- Nanas JN, et al: Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol 2006; 48(12): 2485-2489.
- Ezekowitz JA, McAlister FA, Armstrong PW: Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12,065 patients with new-onset heart failure. Circulation 2003; 107(2): 223-225.
- Anker SD, et al: Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25): 2436-2448.
- Swedberg K, et al: Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13): 1210-1219.
- Sica DS: Pharmacotherapy in congestive heart failure: ACE inhibitors and anemia in congestive heart failure. Congest Heart Fail 2000; 6(6): 330-332.
- McMurray JJ, et al: ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14(8): 803-869.
- Ganzoni AM: Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr 1970; 100(7): 301-303.
- Moore RA, et al: Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord 2011; 11: 4.
- Bolger AP, et al: Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol 2006; 48(6): 1225-1227.
- Toblli JE, et al: Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50(17): 1657-1665.
- Okonko DO, et al: Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008; 51(2): 103-112.
CARDIOVASC 2014; 13(3): 4-7