Once again, the European Lung Cancer Conference was held in Geneva. Once again, the focus was primarily on non-small cell lung cancer. Therapies targeting mutated and non-mutated variants of EGFR are being explored alone or in combination with chemotherapy. Several of these drugs are already approved in the EU and the USA. Furthermore, with a therapy in the field of mesothelioma, an innovative approach from immunology was up for discussion.
Things are happening in the treatment of non-small cell lung cancer (NSCLC). On the one hand, study results on osimertinib were discussed at the congress. This is a potent third-generation irreversible EGFR tyrosine kinase inhibitor that has already been approved in the US and EU (not yet in Switzerland). Two late-breaking abstracts confirmed its efficacy in EGFR-mutated NSCLC, particularly in the presence of a T790M mutation. An update from AURA P1 (phase I) and pooled data from AURA extension and AURA 2 (both phase II trials) showed the following results for osimertinib at the recommended dose of 80 mg/d (oral):
- Of the 63 patients from AURA P1, 71% were found to have an objective response that lasted a median of 9.6 months. Progression-free survival was 9.7 months. The most common causal adverse events included rash (37%, none of grade 3) and diarrhea (35%, of which 2% were grade 3).
- Of the 411 patients in the two pooled phase II trials, 66% had an objective response (as assessed by independent review) that lasted a median of 12.5 months. Progression-free survival was 11 months. After one year, 47.5% of participants were still progression-free. Again, rash (41%, including 1% grade 3) and diarrhea (38%, including 1% grade 3) were among the most common causal adverse events.
The pooled phase II data clearly confirm the results from AURA P1 and previous publications, the presenters said. In patients with advanced NSCLC and the EGFR T790M mutation who have progressed on prior EGFR TKI therapy, osimertinib 80 mg/d provides a high response rate over a relatively long duration with encouraging progression-free survival (especially compared to the four to five months with chemotherapy) and a manageable side effect profile.
According to the authors, molecular diagnosis of the T790M mutation should be standard by now. The data would clearly show that patients with an appropriate resistance mechanism benefit from osimertinib.
Osimertinib also effective in the first-line setting
The second presentation showed data from two phase I cohorts with osimertinib at doses of 80 or 160 mg/d, this time administered as first-line therapy, in 60 therapy-naïve patients. Again, this was an update from the AURA study. Patients had locally advanced or metastatic EGFR-mutated NSCLC (five of whom were T790M-positive). Median follow-up was 16.6 months. The overall objective response rate was 77% (67% in the 80 mg cohort and 87% in the 160 mg cohort). Patients remained progression-free for a median of 19.3 months. After one and a half years, a total of 55% resp. No progression in 57% (80 mg) and 53% (160 mg), depending on cohort.
The most common adverse events were diarrhea (60% and 87%, respectively, of which 0% and 7% were grade 3 or higher), stomatitis (43% and 50%, respectively, of which 0% and 3% were grade 3 or higher), and paronychia (30% and 53%, respectively, of which 0% and 7% were grade 3 or higher). At 10% (80 mg) resp. 47% (160 mg) of patients required dose reduction to control side effects.
Does taking osimertinib change tumor biology?
Osimertinib also showed potential in the first-line setting, the authors concluded. It is one of the best overall response rates achieved with first-line therapies for EGFR-mutated NSCLC, and progression-free survival also far exceeds experience with corresponding first- and second-generation drugs (approximately 10-13 months). Many patients have not yet shown progression and continue to benefit from osimertinib.
In addition, in those with progressive disease, the T790M mutation does not appear to be responsible for resistance, initial data indicate. It is possible that first-line osimertinib thus alters tumor biology. The safety profile is good, especially at the lower (approved) dose, and a rate of 10% patients with dose reductions is considered low.
Further studies in preparation
EGFR inhibition is currently the standard of care for NSCLC patients with EGFR-activating mutations. Via the T790M mutation, 50-60% of patients develop resistance to treatment. Osimertinib is particularly helpful for these patients because it potently inhibits the original EGFR mutations (exon 19 and 21), but also the EGFR T790M mutation. Osimertinib can delay resistance, and the tumor must apparently seek new resistance mechanisms in addition to the EGFR T790M mutation. Clarity on the benefits of the third-generation inhibitor in the first-line setting is now expected to come from a phase III trial of more than 500 patients comparing osimertinib with erlotinib and gefitinib. Results are expected in about a year and a half.
Necitumumab – patients with EGFR-expressing tumors benefit most
In addition to osimertinib, there were also new data on necitumumab. This monoclonal antibody also targets EGFR and is administered along with chemotherapy (gemcitabine and cisplatin) in NSCLC patients with advanced squamous cell carcinoma. Necitumumab is already approved in the USA and the EU.
The data presented at the congress were from the SQUIRE trial (phase III) and addressed the subgroup of patients with EGFR-expressing tumors (95% of the evaluated population [n=982]). While there was no benefit in the 5% patients without EGFR protein, the 95% with EGFR-expressing tumors were found to improve overall and progression-free survival by a significant 21% and 16%, respectively (compared with chemotherapy alone) – a slightly greater benefit than in the overall population. Given the mechanism of action of necitumumab, this is an obvious result: where there is no receptor and thus no target, the drug cannot bind. According to the EMA, necitumumab is therefore approved only for patients with EGFR-expressing tumors, while the FDA decided based on the overall SQUIRE population and did not further specify approval.
Even this subgroup analysis does not allow a firm conclusion, because the group of patients without EGFR-expressing tumors was clearly too small (5% resp. only 47 patients) and the study design did not target these patients. Larger studies must await to say with certainty whether or not patients with EGFR-negative tumors will also benefit from necitumumab.
Does a bacterium help against mesothelioma?
Malignant pleural mesothelioma is a rare but very aggressive disease with poor prognosis. Treatment is difficult and currently consists of standard pemetrexed and platinum chemotherapy. Response rates of about 30% are achieved, the benefit in survival is thus small. New treatment approaches are therefore needed. One such promises immunotherapy with a live bacterium called CRS-207, an attenuated form of Listeria monocytogenes with two gene deletions, to reduce pathogenicity. The bacterium was engineered to express mesothelin, an antigen that is overexpressed by various tumors, including precisely mesothelioma, and is important for cell survival. Thus, CRS-207 is expected to induce an anti-mesothelin response and thus an endogenous, adaptive, tumor-specific immune response. Together with chemotherapy, the tumor microenvironment is altered and immune-mediated killing of tumor cells is enabled.
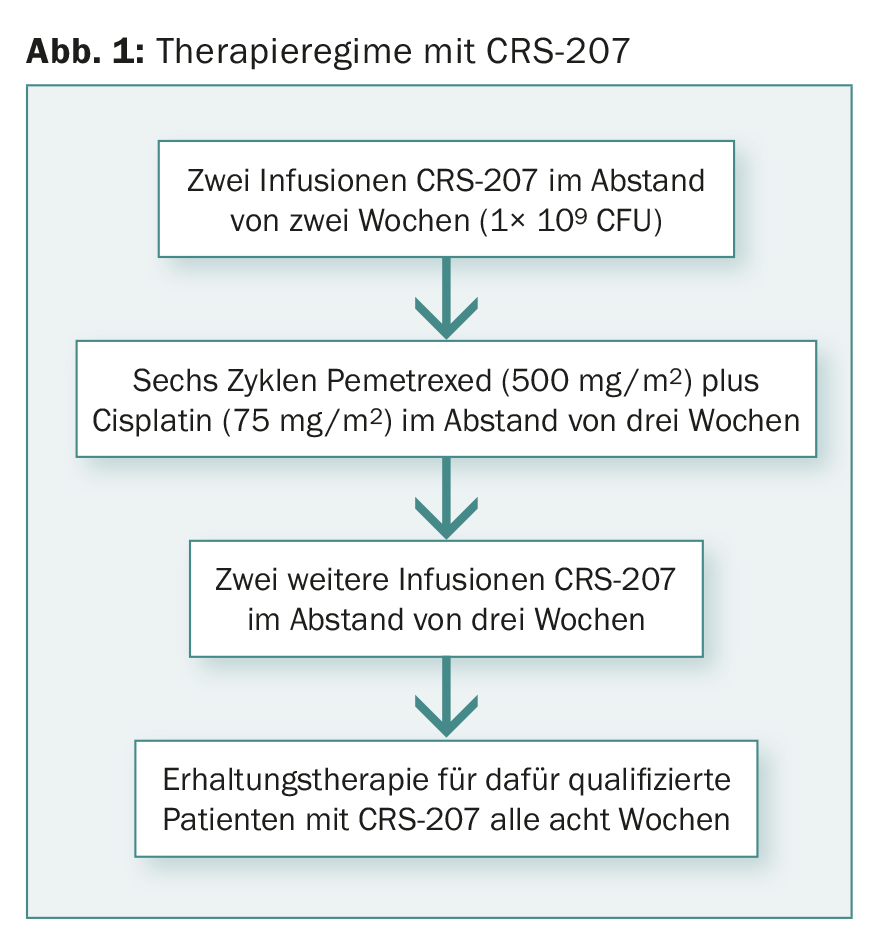
Chemotherapy-naïve patients with advanced, unresectable plauramesothelioma participated in the phase Ib trial. At the time of presentation, data from 38 patients had been evaluated. Figure 1 shows the therapy regime.
Immunohistochemistry found an increase in tumor-infiltrating lymphocytes after CRS treatment. After a median follow-up of 9.4 months, 59% of those treated showed a partial response. In 35%, the disease was stable. This adds up to a disease control rate of 94%. On median, patients lived 8.5 months progression-free.
No serious side effects or deaths were associated with the treatment. Fever, temperature drops/rigor, hypotension, nausea, and vomiting were reported most frequently (grade 1 and 2). Most side effects were related to the infusion itself and resolved after 24 hours.
Immune response is actually stimulated
A response rate of nearly 60% with an overall disease control rate of more than 90% was encouraging, the presenters summarized the data. Furthermore, the new approach measured not only tumor-infiltrating lymphocytes, but also corresponding changes in circulating immune cells and serum biomarkers. For example, one measured an increase in macrophages, infiltrating CD8+ cells, and natural killer cells. The targeted specific and non-specific immune response with subsequent synergistic targeting of the tumor in combination with chemotherapy could thus indeed be initiated.
The therapy will now be further investigated in phase III trials, as it appears to provide significantly better results than chemotherapy alone. The safety and tolerability profile of the new approach is also surprisingly good, with no cumulative toxicities appearing to occur.
Source: ELCC 2016 European Lung Cancer Conference, April 13-16, 2016, Geneva.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(4): 30-32.