Patients with active rheumatoid arthritis often suffer from considerable pain. Therefore, pain control is an important treatment goal in this patient group. In a post-hoc analysis of the FINCH studies 1-3, the specific effects of the JAK1 inhibitor filgotinib on pain control and the relationship between efficacy and pain response were investigated.
Rheumatoid arthritis (RA) is often accompanied by significant pain, which can severely affect patients and reduce their quality of life. It is the most commonly reported first RA symptom and the most common reason for patients with RA to see a doctor. Several mechanisms may be responsible for the pain experienced by RA patients: it may be directly related to disease activity, but non-inflammatory mechanisms such as peripheral and central sensitization may also be involved. Consequently, patients may still suffer from a significant pain burden even when inflammation is under control, as assessed by composite disease activity scores that meet the criteria for remission or low disease activity on RA treatment. This may even be the case in patients with early RA who have achieved optimal disease control according to treatment guidelines.
While strong analgesics can be prescribed for pain management, opioids are often associated with an unfavorable risk-benefit ratio. Ideal medications for the treatment of rheumatoid arthritis would allow target levels of disease activity to be achieved and would also have an additional benefit in terms of patient-reported pain.
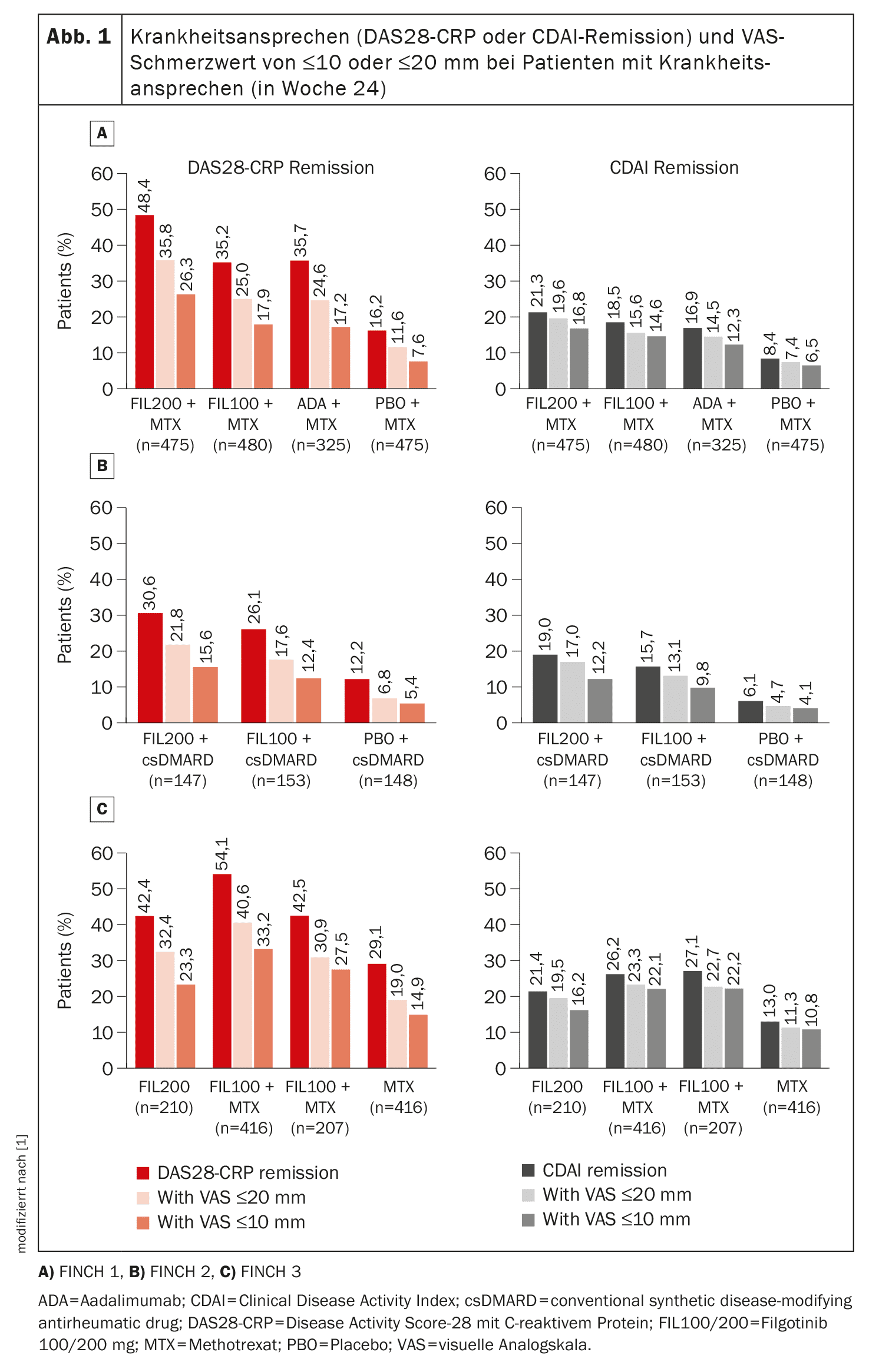
Professor Dr. Peter C. Taylor from the Botnar Research Centre, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, and colleagues evaluated FINCH trials 1, 2 and 3 [1]. In the randomized, double-blind phase 3 trials, filgotinib 100 mg or 200 mg was used in patients who responded inadequately to methotrexate (FINCH 1) or bDMARDs (FINCH 2) or who were methotrexate-naïve (FINCH 3). Each study enrolled patients aged ≥18 years with active moderate-to-severe RA (defined as ≥6 swollen joints and ≥6 painful joints). Assessments included: residual pain response of ≤10 and ≤20 mm on a 100 mm visual analog scale (VAS) and the proportion of patients achieving a VAS pain response in addition to remission or low disease activity (according to Disease Activity Score 28 with C-reactive protein (DAS28-CRP) or Clinical Disease Activity Index (CDAI) criteria).
Filgotinib reduced signs and symptoms of RA with an acceptable safety profile
The results of the analysis indicate that filgotinib reduces pain in patients with active RA who had an inadequate response to methotrexate or bDMARDs or who were methotrexate-naïve.
The effect of filgotinib was rapid: A reduction in VAS pain scores was seen as early as week 2, and the effect was sustained over a longer period of time (up to week 52 in FINCH 1 and 3 and up to week 24 in FINCH 2) (Fig. 1).
Improvements from baseline were significantly greater with filgotinib than with placebo, with the greatest improvements observed in patients receiving filgotinib 200 mg plus methotrexate. A reduction in pain (by 30%, 50%, 70% and 90%) was generally achieved earlier with filgotinib 200 mg than with adalimumab and sooner with filgotinib 200 mg or 100 mg than with placebo or methotrexate. For example, the HR for a 30% reduction in pain was 1.16 for filgotinib 200 mg vs. adalimumab (95% CI 1.00-1.35, p=0.034). Similarly, the median time in which the VAS pain score was ≤10 or ≤20 mm was about 3 weeks longer with filgotinib 200 mg than with adalimumab and was longer with each filgotinib dose than with placebo or methotrexate, the authors explain.
The improvement observed with filgotinib 200 mg versus adalimumab is likely to be clinically relevant and meaningful to patients, Prof. Taylor and colleagues said.
In addition, the finding that filgotinib 100 mg and adalimumab result in comparable pain outcomes emphasizes the value of the lower dose of filgotinib in patients who achieve disease activity control that includes satisfactory pain improvement.
Current treatment guidelines advocate a treat-to-target approach to RA management, with therapeutic approaches modified until disease remission or low disease activity is achieved.
Inhibition of JAK 1 and 2 has been shown to relieve pain in patients with rheumatoid arthritis.
Since JAK inhibitors reportedly do not cross the blood-brain barrier, their effect on pain may be triggered by pain-mediating cytokines (such as granulocyte-macrophage colony-stimulating factor and interleukin-6) rather than by direct effects on the central nervous system.
Baricitinib was found to relieve pain to a greater extent than adalimumab, although both therapies had a similar effect on clinical inflammatory parameters.
This suggests that JAK inhibition may offer potential added value for pain relief if treat-to-target goals are otherwise achieved.
Similarly, in the analysis by Prof. Taylor et al. in patients with active RA who had an inadequate response to methotrexate, a greater proportion of patients treated with filgotinib 200 mg vs. adalimumab and a similar proportion of patients treated with filgotinib 100 mg vs. adalimumab achieved a significant response to pain in addition to clinical response (remission or low disease activity). In the assessment of remission, this finding was more pronounced when DAS28-CRP rather than CDAI criteria were used – an observation also reflected in the results of multivariable analyses, which showed that baseline DAS28-CRP score, but not CDAI score, predicted the achievement of VAS pain scores of ≤10 or ≤20 mm.
Their analysis of the FINCH studies shows that filgotinib has a rapid and long-lasting effect on pain that is greater than or comparable to the effect of active comparator therapies in all RA patient groups, the authors summarize. Compared to adalimumab, the effects on pain with filgotinib 200 mg were generally more favorable and comparable to those of filgotinib 100 mg.
Take-Home-Messages
- With filgotinib, significantly greater pain reduction was observed as early as week 2 in RA patients who had an inadequate response to methotrexate or bDMARD or were methotrexate-naïve vs. placebo, adalimumab and methotrexate, and the time to 30%, 50%, 70% or 90% pain reduction was generally shorter with filgotinib.
- During the 52-week study period, patients who received filgotinib 200 mg together with methotrexate achieved an additional 3 weeks in which the pain score on the visual analog scale was ≤10 mm compared to those who took adalimumab together with methotrexate.
- The effects of filgotinib 100 mg plus methotrexate on pain were similar to those in the group with adalimumab plus methotrexate.
Literature:
- Taylor PC, Kavanaugh A, Nash P, et al: Impact of filgotinib on pain control in the phase 3 FINCH studies. RMD Open 2024; 10: e003839; doi: 10.1136/rmdopen-2023-003839.
InFo RHEUMATOLOGIE 2024; 6(1): 20-22