St. John’s wort extracts are proven for the treatment of depressive disorders. However, some preparations, by inducing CYP3A4 and P-glycoprotein (ABCB1), lower the plasma levels of other drugs and impair their efficacy. These pharmacokinetic interactions are not observed with hyperforinergic extracts. Treatment with such extracts increases the safety of this therapy.
According to the WHO, approximately 264 million people of all age categories in the world suffer from depression. It represents the main cause of incapacity for work [1]. International and national treatment guidelines recommend the use of psychotherapy or pharmacotherapy for mild depression, and psychotherapy or/and pharmacotherapy for moderate and severe depression [2]. In addition to synthetic antidepressants such as SSRIs, phytotherapeutics such as St. John’s wort (Hypericum perforatum) are also part of the therapeutic toolbox, after its efficacy and good tolerability have been demonstrated in numerous studies [3–5]. However, St. John’s wort should also be taken under expert medical supervision [6]: although there are numerous over-the-counter preparations on the market in addition to those available by prescription, St. John’s wort is known for its pharmacokinetic interaction potential with a large number of drugs, which is why its use is not without risk. This phytopharmaceutical achieved a disturbing notoriety after it became known that heart transplant patients treated with ciclosporin who received comedication with St. John’s wort for depressive comorbidity experienced rejection of the transplanted organ [7]. Hypericum extract induces CYP3A4, the enzyme responsible for the metabolism of ciclosporin. The efficacy of this immunosuppressor is considerably reduced by the decrease in its biodisponibility [8]. The component of St. John’s wort responsible for the induction of CYP3A4 is hyperforin, although hypericum extracts available on the market vary considerably in the content of this compound.
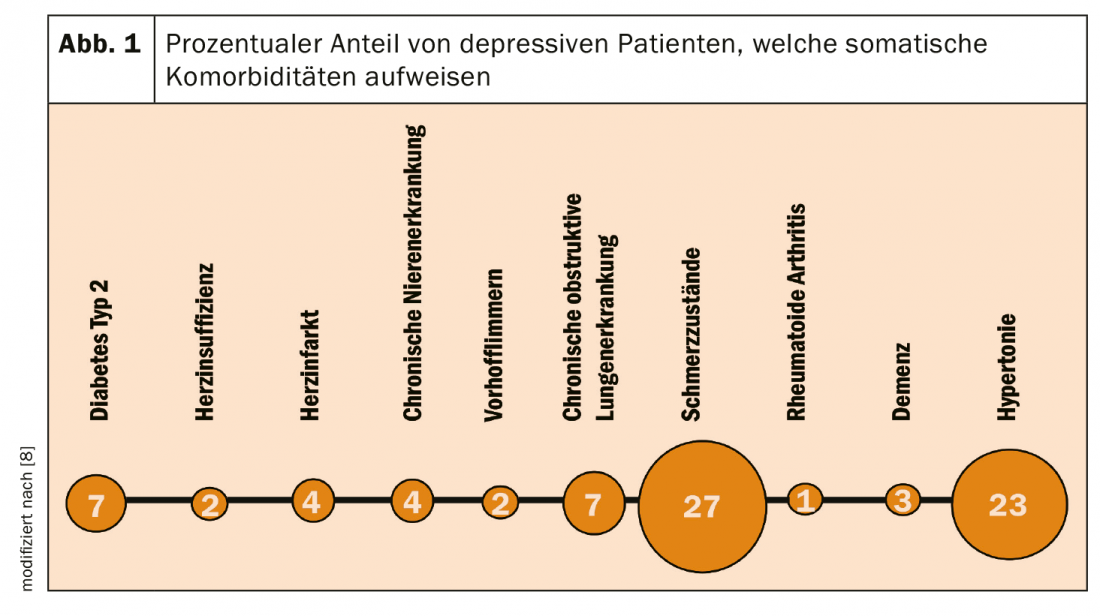
Depressed patients are treated not only by a specialist in psychiatry and psychotherapy, but also by a specialist in general medicine or internal medicine. Depression is at particularly high risk for certain comorbidities (Fig. 1), but depressed patients also suffer from other somatic and mental illnesses that cause comedications [8]. Fortunately, there is a low-hyperfornin extract that is characterized by a low risk of interaction with other drugs, favoring conditions of safe therapy in these patients.
Hypericum extracts and their indications
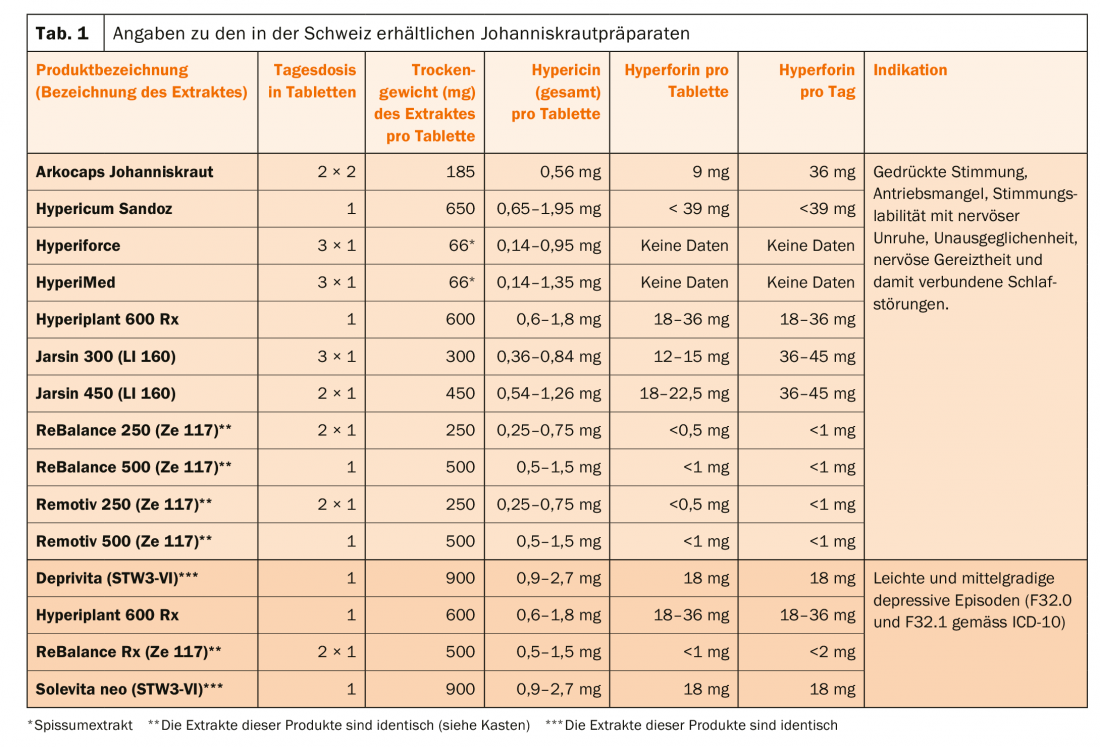
The hypericum extracts discussed in this article are recommended for two different diagnostic groups, depending on the preparation (Table 1):
- For patients who suffer from depressed mood, mood instability, inner restlessness, states of tension and associated problems falling asleep and staying asleep. These are drugs of dispensing category D.
- For patients diagnosed with a mild or moderate depressive episode (F32.0 and F32.1 according to ICD-10). These are drugs of dispensing category B.
The daily doses for the treatment of patients with St. John’s wort differ significantly (Table 1). Now, however, an analysis of 28 clinical trials of the efficacy of various Hypericum extacts in major depression shows that at doses ≥180 mg/day extract, an efficacy plateau is achieved, in that even with doses of 1800 mg/day, no better treatment results were achieved [9].
Hypericum has relatively few side effects and is well tolerated [10]. Some degree of photosensitization applies to all Hypericum phytotherapeutics, and heavy sun exposure should be avoided. However, the risk appears to become clinically significant only at daily doses of >2-4 g hypericum (corresponding to 5-10 mg/day hypericin) [9].
Pharmacodynamic interactions must also be considered. Hypericum is also a serotonin uptake inhibitor and therefore its combination with SSRIs carries the risk of serotonin syndrome, although in the case of citalopram, for example, the expected induction of its metabolism to a lowering of its biodisponibility would make such a risk less likely [10].
Mechanism of action
Hypericum extracts contain a large number of active substances, the concentrations of which vary not only between preparations, but also in the individual preparation, e.g. for total hypericin it varies between 0.14-1.35 mg in one film-coated tablet Hyperimed® (Tab.1). Previously, hypericin, pseudohypericin (where hypericin + pseudohypericin are referred to as total hypericin), and hyperforin were thought to be mainly responsible for the therapeutic effects, but it has now been shown that low-hyperforin extracts are also effective. Hypericum contains other compounds such as those of the flavinoid type (quercetin, quercetrin, rutin), which may also contribute to the therapeutic effect [11]. In general, the extracts inhibit the reuptake of serotonin, norepinephrine and dopamine, but they also act on GABA and glutamate. Their action profile is similar to that of many synthetic antidepressants, including the ability to cause downregulation of β-receptors and upregulation of 5-HT2 receptors (i.e., the number of receptors decreases and increases, respectively). Hyperforin favors the entry of Na and Ca ions into TRPC6 (transient receptor potential channels) channels. In animal experiments, depending on the model, hyperforin and/or hypericin prove to be active [12,13]. Nevertheless, it is difficult to draw conclusions regarding a plant mechanism, since the standardization of extracts is based on only one or two compounds. Low-hyperforin extracts are those containing <1 mg/dose of hyperforin, as opposed to high-hyperforin products (>1 mg/dose) (Table 1) (Box).
Clinical pharmacokinetics and metabolism
In a pharmacokinetic study, a Tmax of 5-10h, and an elimination half-life of 12-28h were measured for hypericin. Corresponding measurements were also obtained for pseudohypericin (1.5-4h; 5-38h), hyperforin (3-8h; 10-28h), and quercitin (0.5-6h (with 2 maxima in this period!); 0.8-7h) [14]. Studies on the role of enzymes participating in the metabolism of hyperforin and hypericin are rare. At least 57 metabolites are formed from hyperforin, with forms of CYP2C and CYP3A playing a role in their formation. It inhibits CYP2D6 and CYP3A in vitro, but it is not clear to what extent this finding is clinically relevant, as the inductive effect predominates after prolonged administration [15]. Thus, other drugs such as ritonavir also inhibit CYP3A4 in an initial step before the inductive effect on the same enzyme comes to the fore.
CYP3A4 is responsible for the metabolism of nearly half of all drugs. It shares numerous substrates with the P-glycoprotein (PgP; ABCB1), which acts as an efflux transporter to transport drugs out of the cell. Hyperforin contained in hypericum extracts binds to pregnane X receptor (PXR), after which CYP3A4 as well as PgP are induced in the liver, intestine, and other organs via various steps [9]. Less pronounced and, more importantly, not yet accepted are findings that hyperforin also substantially induces CYP1A2, CYP2C9, and CYP2C19.
Induction of CYP3A4 and PgP may occur as early as 3 days of treatment. It reaches a maximum after approx. 1-2 weeks, although it can still be observed for a similarly long period after interruption of treatment, i.e. it is after all reversible [16]! This circumstance must therefore also be taken into account when switching from a hyperforin-rich to a hyperforin-poor preparation.
Pharmacokinetic interactions of hyperforin-rich hypericum extracts.
Hyperforin-rich hypericum extracts therefore promote the metabolism of many drugs, as has already been described using the example of ciclosporin. However, they also prevent the transport of PgP substrates, for example the uptake of digoxin from the intestine into the blood. Because many steroids are metabolized via CYP3A4, there is a risk of bleeding or even pregnancy with hormonal contraception with, for example, ethinylestradiol or desogestrel during concomitant treatment with hyperforin-rich Hypericum.
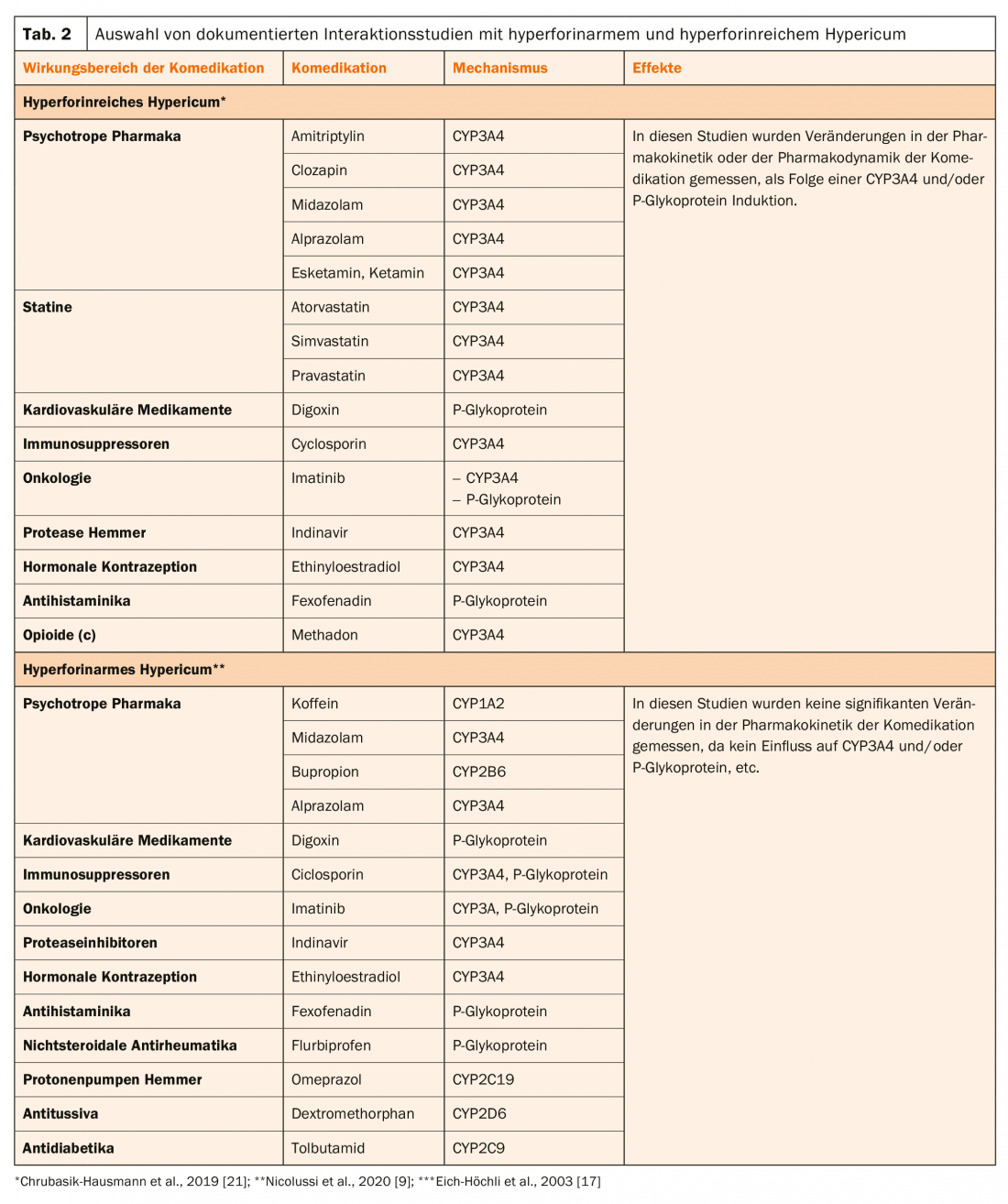
For example, the SPC of Hypericum Sandoz® lists various comedications as contraindications, such as certain immunosuppressants (e.g. ciclosporin, tacrolimus or sirolimus), anti-HIV drugs from the group of non-nucleoside reverse transcriptase inhibitors (e.g. nevirapine) and proteinase inhibitors (e.g. indinavir), certain cytostatic drugs (e.g. imatinib, irinotecan) and oral anticoagulants of the coumarin type (Tab. 2).
In patients treated with methadone, additional antidepressant treatment with St. John’s wort caused not only a decrease in its plasma levels but also withdrawal symptoms, which regressed only after interruption of hypericum treatment [17].
Esketamine has just been introduced in Switzerland as an adjunctive antidepressant for treatment-resistant patients. Since it is degraded by CYP3A4, it is not surprising that hyperforin-rich Hypericum decreases its plasma levels by about half [18]. It may be assumed that this will not be the case with hyperforinar St. John’s wort.
Comparison of hyperforin-poor with hyperforin-rich Hypericum.
The pharmacokinetic interactions of Hypericum extracts have been documented in numerous studies and reviews [9,19–21].
Currently, there is only extract Ze 117, which is low in hyperforin according to EMA guidelines (Box, Table 1) . Thus, after administration of this product, no decrease in digoxin plasma levels is observed in subjects treated with this drug [22]. Table 2 presents interaction studies with hyperforin-poor and hyperforin-rich Hypericum. From this, it is clear that these extracts clearly differ in their effects on CYP3A4 and/or P-glycoprotein. Thus, treatment with these extracts is likely to be associated with higher risks as a result of pharmacokinetic interactions, which manifest themselves primarily as a loss of clinical effect of the comedication.
| The EMA (European Medicines Agency) approved in 2018 that the so-called SPC (Summary of Product Characteristics) no longer need to include a warning about pharmacokinetic interactions if in the daily dose of a hypericum extract the hyperforin content is <1 mg/day, as is the case with extract Ze 117. Switzerland (Swissmedic) was the first country to adopt this. Such warnings have already been removed from the drug information on Ze 117 hypericum extracts Remotiv 250/500 and ReBalance 250/500, but the rewrite procedure is still pending for ReBalance Rx, so this product is not currently supplied by the company (as of March 2020). |
Practical consequences and conclusions
The range of Hypericum phytotherapeutics is confusing. For example, in the case of hyperforinar Ze 117 extract, three products are offered which are identical, namely Remotiv®, Rebalance®, Rebalance®Rx. The latter two products are reimbursed by the cashier, while Remotiv is in the OTC line and is not reimbursed. As mentioned above, Rebalance® RX (Schedule B) must be prescribed by a physician for mild to moderate depression. Rebalance and Remotiv, on the other hand, are on Schedule D, and therefore can be dispensed by pharmacies and drugstores for the treatment of depressed mood, mood instability, etc. Now, however, the drug information for Rebalance® RX does not yet specify that this is a product with a maximum of 0.1 mg hyperforin (per daily dose), but it does for Remotiv® and Rebalance® (box) . It is therefore important that the cooperation between physician – pharmacist – patient is optimal, especially since it must be made clear to the patient that automedication can be associated with risks when, for example, switching from one Ze 117 extract to another product, especially because the majority of the other products are rich in hyperforin. When switching from a hyperforin-rich to a hyperformin-deficient extract, the dose of comedication may need to be carefully adjusted, in which case therapeutic drug monitoring would be recommended [23,24]. With certain caveats, low hyperforin St. John’s wort extracts are now considered effective and safe medications for the treatment of mild to moderate depression. There are not only many patients who prefer phyotherapy, but also doctors who would like to use it specifically, i.e. patient-related.
Literature:
- www.who.int/news-room/fact-sheets/detail/depression (last access on 15.4.2020)
- Holsboer-Trachsler E, Hättenschwiler J, Beck J, et al: The acute treatment of depressive episodes. Swiss med forum. 2016;16(35): 716-725.
- Apaydin EA, Maher AR, Shanman R, et al: A systematic review of St. John’s wort for major depressive disorder. Syst Rev. 2016;5(1): 148.
- Rahimi R, Nikfar S, Abdollahi M: Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1): 118-127.
- Linde K, Berner MM, Kriston L: St John’s wort for major depression. Cochrane Database Syst Rev. 2008(4): CD000448.
- Anheyer D, Haller H, Klose P, et al: Herbal medicines for psychiatric disorders. Nervenarzt. 2018;89(9): 1009-1013.
- Ruschitzka F, Meier PJ, Turina M, et al: Acute heart transplant rejection due to Saint John’s wort. Lancet. 2000;355: 548-549.
- Dumbreck S, Flynn A, Nairn M, et al: Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350: h949.
- Nicolussi S, Drewe J, Butterweck V, et al: Clinical relevance of St. John’s word drug interactions revisited. Br J Pharmacol. 2020;177(6): 1212-1226.
- Henderson L, Yue QY, Bergquist C, et al: St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. British Journal of Clinical Pharmacology. 2002;54(4): 349-356.
- Gurley BJ, Swain A, Hubbard MA, et al: Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John’s wort, and Echinacea. Mol Nutr Food Res. 2008;52(7): 755-763.
- Muller WE: Current St John’s wort research from mode of action to clinical efficacy. Pharmacol Res. 2003;47(2): 101-109.
- Leuner K, Kazanski V, Muller M, et al: Hyperforin – a key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J. 2007;21(14): 4101-4111.
- Schulz HU, Schurer M, Bassler D, Weiser D: Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin following single and multiple oral dosing of a hypericum extract containing tablet. Drug discovery. 2005;55(1): 15-22.
- Hokkanen J, Tolonen A, Mattila S, Turpeinen M: Metabolism of hyperforin, the active constituent of St. John’s wort, in human liver microsomes. Eur J Pharm Sci. 2011;42(3): 273-284.
- Johne A, Schmider J, Brockmöller J, et al: Decreased Plasma Levels of Amitriptyline and Its Metabolites on Comedication With an Extract From St. John’s Wort ( Hypericum perforatum ). Journal of Clinical Psychopharmacology. 2002;22(1): 46-54.
- Eich-Höchli D, Oppliger R, Powell Golay K, et al: Methadone maintenance treatment and St. John’s wort – A case report. Pharmacopsychiatry. 2003;36: 35-37.
- Peltoniemi MA, Saari TI, Hagelberg NM, et al: St John’s wort greatly decreases the plasma concentrations of oral S-ketamine. Fundam Clin Pharmacol. 2012;26(6): 743-750.
- Soleymani S, Bahramsoltani R, Rahimi R, Abdollahi M: Clinical risks of St John’s Wort (Hypericum perforatum) co-administration. Expert Opin Drug Metab Toxicol. 2017;13(10): 1047-1062.
- Zahner C, Kruttschnitt E, Drewe J, et al.: No clinically relevant interactions of st. john’s wort extract ze 117 low in hyperforin with cytochrome p450 enzymes and p-glycoprotein. Clinical Pharmacology & Therapeutics. 2019;106(2): 432-440.
- Chrubasik-Hausmann S, Vlachojannis J, McLachlan AJ: Understanding drug interactions with St John’s wort (Hypericum perforatum L.): impact of hyperforin content. J Pharm Pharmacol. 2019;71(1): 129-138.
- Mueller SC, Uehleke B, Woehling H, et al: Effect of St John’s wort dose and preparations on the pharmacokinetics of digoxin. Clin Pharmacol Ther. 2004;75(6): 546-557.
- Hiemke C, Bergemann N, Clement HW, et al: Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry. 2018; 51(1/2): 9-62.
- Klein HG, Haen E: Pharmacogenetics and therapeutic drug monitoring. Berlin, Boston: De Gruyter; 2018. 465 p.
InFo NEUROLOGY & PSYCHIATRY 2020; 18(3): 20-23.
GP PRACTICE 2020; 15(6): 38-41