If there is a change in character or new onset of motor clumsiness, measure head circumference and perform neurological examination, possibly repetitively. In Switzerland, the treatment of childhood brain tumors is part of Highly Specialized Medicine (HSM). Optimal treatment and care of children with brain tumors is only possible at hospitals with experienced and well-coordinated interdisciplinary teams.
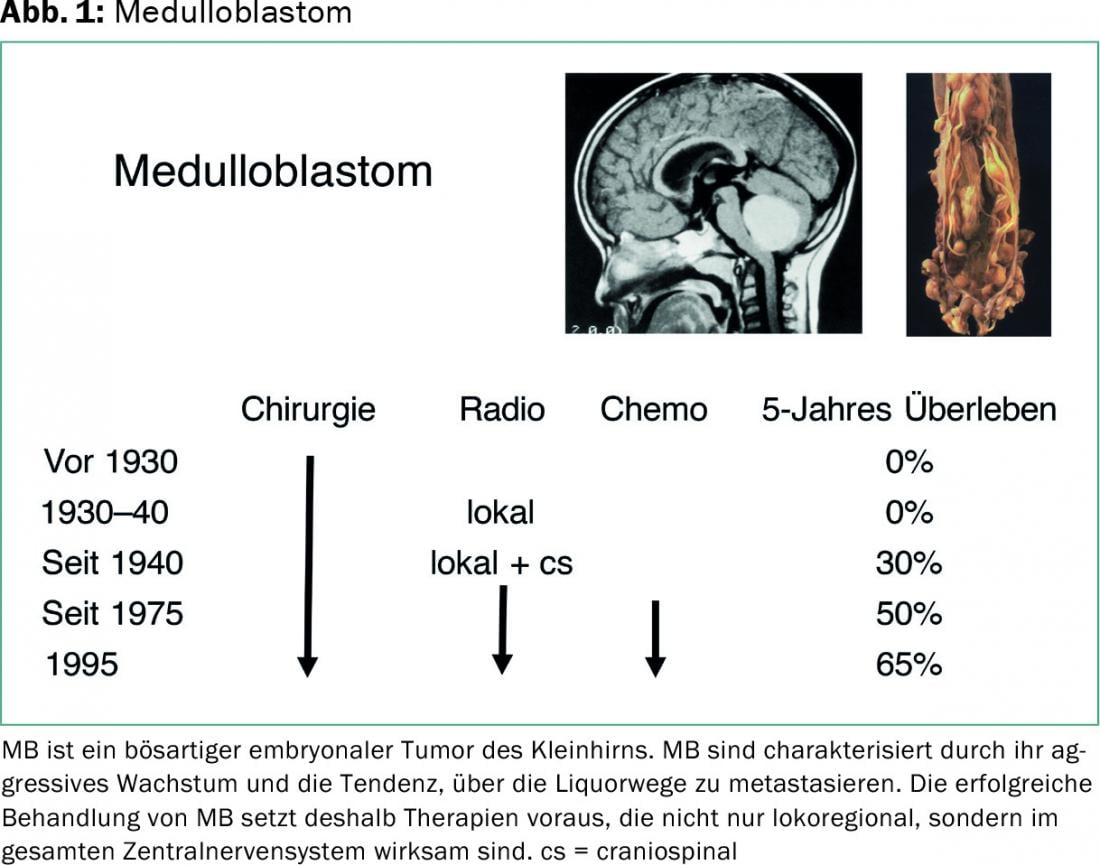
Medulloblastoma (MB) is a malignant embryonal tumor of the cerebellum. It occurs mainly in infancy and childhood and is the most common malignant brain tumor in this age group. According to the WHO classification of tumors of the central nervous system, MB are classified as grade IV.
MB are characterized by their aggressive growth and tendency to metastasize via the CSF pathways. Successful treatment of MB therefore requires therapies that are effective not only locoregionally but throughout the central nervous system. After tumor resection, which should be as complete as possible, these are usually radiotherapy (with irradiation of the entire brain and spinal axis, as well as saturation in the area of the extended tumor region), and chemotherapy.
MB often manifest with cerebellar signs and symptoms of increased intracranial pressure. Due to the location in the posterior fossa, there is an obstruction of CSF outflow pathways, resulting in hydrocephalus occlusivus. In children under two years of age, intracranial pressure is manifested mainly by excessive head growth and bulging fontanelles due to the still open sutures and fontanelles. Early recognition of intracranial pressure is difficult when it is initially manifested only by a change in temperament. Here, it is important for the practitioner to measure the head circumference, as excessive head growth is often not noticed by the parents, and to perform neurological examinations, possibly repetitively.
The perception and correct interpretation of sometimes very discrete motor disorders (“He’s just a bit clumsy”) can also be difficult. In older children, adolescents, and adults, headache, vomiting, abducens paresis (often not subjectively bothersome), and congestive papules predominate.
Most often, MB develops sporadically, with no identifiable familial or environmental causes. In a few cases, MB occurs in the context of familial cancer predisposition syndromes, for example, Li-Fraumeni syndrome.
Survival rates of MB patients
Survival rates of MB patients have improved in recent decades. While only about 20% of patients survived before 1970, five-year survival rates are much higher today at 65%. However, depending on clinical, histologic, and molecular factors, prognosis varies widely in individual cases [1].
Unfortunately, 40-100% of MB long-term survivors were found to have growth and endocrine dysfunction, some of which was considerable, and nearly 90% had cognitive deficits (including problems with concentration and memory). The cognitive deficits are usually not a direct consequence of the tumor or tumor surgery, but are primarily related to radiotherapy, depending on the radiation dose and the age of the patient. Normally, this does not result in a loss of what has already been learned, but in a reduction of the learning rate.
Hearing disorders are also relatively common: Platinum derivatives can damage the hair cells in the inner ear, irradiation the auditory nerve. Accordingly, regular audiometries during chemotherapy are important. Depending on the extent of the tumor, MB patients often also suffer from motor dysfunctions incl. Ataxias and paresis. These disorders do not always disappear after therapy. Accordingly, rehabilitation measures such as physiotherapy and occupational therapy should begin during therapy rather than after it has been completed.
Neuropsychological tests are integrated into the therapy-accompanying diagnostics for children. The aim is to promote educational and professional development through appropriate measures. Often, the social integration of former MB patients is also impaired. More than half of MB long-term survivors live alone or in their nuclear families and tend to withdraw because of their handicap.
What strategies are available to improve the obviously significantly impaired quality of life of these patients? In addition to measures of social reintegration and rehabilitation in school and work, these include above all a reduction in therapy-related late effects by avoiding or delaying radiotherapy in young children and better risk-dependent patient stratification.
Forecasting capabilities
Since the early 1980s, several large studies have defined clinical (including imaging) prognostic factors for MB. Detection of leptomeningeal metastases at diagnosis (Fig. 2) was found to be most prognostically significant, followed by residual tumor mass and patient age at diagnosis.
These clinical factors were used to divide MB patients into a “standard risk” group (no metastases, <1.5 cm2 residual tumor mass, patient age at diagnosis ≥3 years) and a “high risk” group (metastases, ≥1.5 cm2 residual tumor mass, and patient age at diagnosis<3 years). However, they are not sufficient to reliably define a group of patients whose risk of tumor progression is so small that they could be treated with significantly less toxic therapeutic modalities.

Serial analysis of gene expression on several hundred MBs has allowed identification of molecular subsets of MB in recent years [2–4].
Currently, MB are classified into four subgroups (Wnt/Wingless=WNT, Sonic hedgehog=SHH, Group 3, Group 4) according to molecular biological criteria (Table 1) . These subgroups also differ in epidemiological and clinical criteria. Evidence of metastasis is found very rarely in the WNT subtype, rarely in the SHH subtype, but frequently in groups 3 and 4. Prognosis is very favorable for the WNT subtype, favorable for infants and intermediate for the elderly in the SHH subtype, poor for the group 3 subtype, and intermediate for the group 4 subtype. Especially in the SHH subtype, new therapeutic options are opened by the use of SMO inhibitors [5].

Current and planned multicenter trials have as goals a reduction in treatment-related late effects while maintaining a high cure rate in low-risk MB, and an improvement in survival rates in high-risk groups [1]. True to the WHO motto, “Cure is more than the eradication of cancer… cure is the restoration of health.”
Literature:
- Gerber NU, et al: Recent developments and current concepts in medulloblastoma. Cancer Treat Rev 2014; 40(3): 356-365.
- Kool M, et al: Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 2012; 123(4): 473-484.
- Northcott PA, et al: Medulloblastomics: the end of the beginning. Nat Rev Cancer 2012; 12(12): 818-834.
- Taylor MD, et al: Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012; 123(4): 465-472.
- Rudin CM, et al: Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 2009; 361(12): 1173-1178.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(5): 25-27.