This year’s ASCO Congress theme was “Collective Wisdom: The Future of Patient-Centered Care and Research.” According to ASCO, in order to continue to make progress in the care and treatment of cancer patients, it is necessary for the various medical disciplines and cancer research to collaborate even more closely and share information and data. We report important study results on glioma, pancreatic cancer, metastatic colon cancer, and lung cancer.
Can adjuvant administration of temozolomide (TMZ) to radiotherapy improve survival of patients with glioma? This question is explored in the CATNON study. Surprising interim results were presented by Martin van den Bent, Erasmus MC Cancer Center in Rotterdam, at the ASCO Congress [1].
Gliomas: adjuvant therapy with temozolomide improves prognosis
The study included 745 patients with anaplastic glioma without 1p19q deletion, which is associated with better prognosis and chemosensitivity. All patients received radiotherapy (RT) with 59.4 Gy in 33 fractions. Randomization was done into four groups:
- RT alone,
- RT and concurrent administration of 75 mg/m2/d TMZ,
- RT followed by 12 cycles of 150-200 mg/m2 TMZ (on days 1-5 per 4 weeks),
- RT with concomitant administration of TMZ followed by 12 cycles of TMZ.
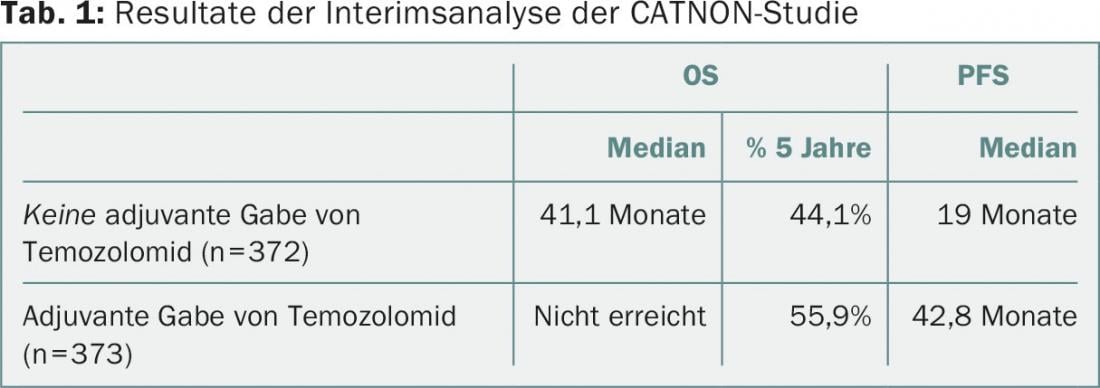
Patients were recruited between December 2007 and August 2015. In October 2015, after a median follow-up of 27 months, the interim analysis was performed. Groups 3 and 4 showed a reduction in hazard ratio (HR) with respect to overall survival (OS) of 0.645, and progression-free survival (PFS) was also significantly prolonged (Tab. 1). “Adjuvant therapy with temozolomide clearly improves survival,” was Dr. van den Bent’s conclusion. Previous studies had shown no benefit for TMZ in OS at five years.
Pancreatic cancer: progress through combination chemotherapy
Which chemotherapy after resection offers the best survival to patients with pancreatic cancer? The results of the ESPAC-4 (European Study Group for Pancreatic Cancer) phase III trial are clear and are expected to lead to a change in treatment guidelines for this usually fatal cancer. In ESPAC-4, patients were first operated on and then randomized into two chemotherapy groups: Group 1 (n=366) received standard therapy with gemcitabine alone (six 4-week cycles of gemcitabine i.v., GEM); Group 2 (n=364) received combination therapy with gemcitabine plus capecitabine orally (GEM/CAP). Patients were recruited between November 2008 and September 2014. The median survival of patients with GEM/CAP was 28 months (95% CI, 23.5-31.5), and that of patients with GEM was 25.5 months (22.7-27.9). The 5-year survival in group 2 was 28.8%, and only 16.3% in group 1.
These results represent a small step forward for the treatment of a group of patients who are in urgent need of better therapeutic options. “Combination chemotherapy should now be the new standard treatment,” opined the study’s first author, John P. Neoptolemos of the University of Liverpool, United Kingdom.
Colon carcinoma: Localization of the primary tumor influences survival rate
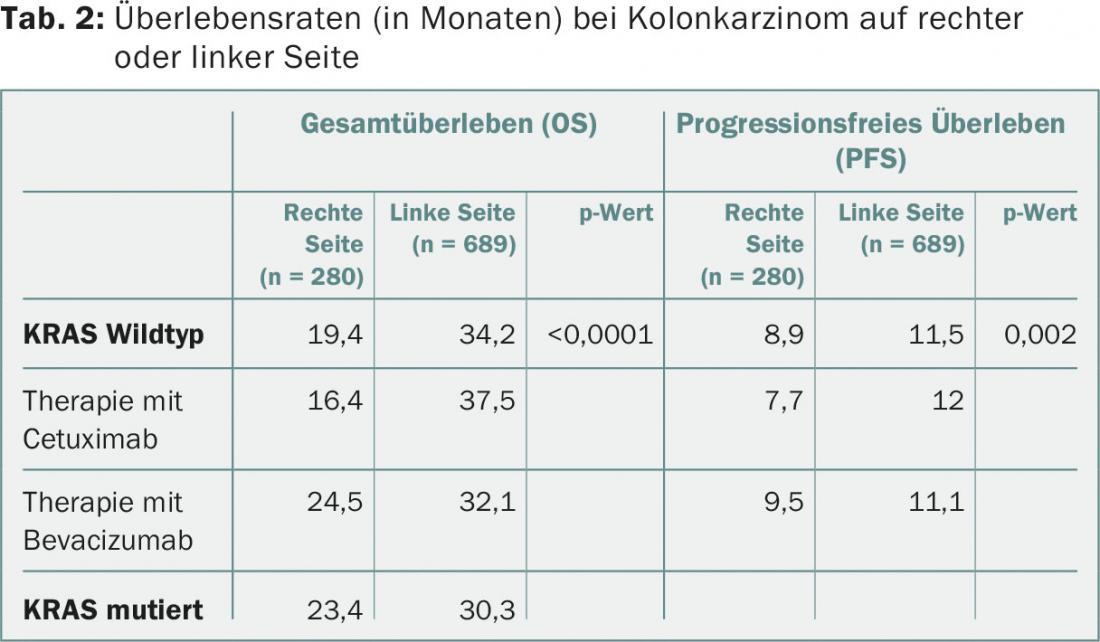
Whether colon cancer affects the right or left side of the colon has a major impact on the patient’s prognosis, according to an analysis by a US study group [3]. Data from the 1137 patients in the CALGB/SWOG 80405 trial (FOLFIRI or mFOLFOX6 with bevacizumab or cetuximab in patients with metastatic colorectal cancer [mCRC] and KRAS wild type) were examined [4]. In 25% of patients, the primary tumor was located in the right side of the intestine (caecum, ascending colon), in 5% in the transverse colon, and in 61% on the left side (descending colon, sigmoid, rectum); in 9% of patients, the location of the primary tumor was unclear.
If the primary tumor was on the left side of the bowel, the median OS was 34.2 months; for primary tumors on the right side, the median OS was only 19.4 months – these results were independent of patient age or sex or previous treatment (Tab.2). OS and PFS were significantly better in left-sided tumors treated with cetuximab and in right-sided tumors treated with bevacizumab (not significant).
“The difference is much larger than we expected,” said first author Alan Venook, MD, University of California, San Francisco, USA. “The results suggest a difference in tumor biology.” These differences are now being investigated. The study authors recommend that future trials for the treatment of mCRC should take into account the different locations of the primary tumor. However, the results obtained so far do not lead to any consequences that would change practice. It should be noted that nowadays study evaluations should take into account the entire RAS mutation status, which was not the case in this study.
Lung cancer: follow-up with the support of an app can prolong life
A web-based app can help patients with lung cancer to detect symptoms of tumor recurrence or complications as early as possible and thus has a significant life-prolonging effect. This is the astonishing result of a Phase III study from France. Included were 121 lung cancer patients at high risk for progression (90% with stage III/IV disease) and with a performance status of 0-2. Patients in the experimental group (n=60) completed a symptom questionnaire once a week using the app on their smartphone, tablet, or computer. Results were sent weekly – between scheduled follow-up appointments – to the treating oncologist. Once defined clinical criteria were met, the oncologist received a notification via email so that he or she could arrange for imaging or another measure. In the control group without app, patients (n=61) were followed up with CT every 3-6 months according to standard. The median follow-up was nine months.
The median OS was 19 months in the experimental group and 11.8 months in the control group. At the time of the first relapse, 81.5% of patients in the experimental study arm had a performance status of 0-1, compared with only 35.3% in the standard arm. The study authors emphasize the benefits of Web-based follow-up: improved survival, better performance status at the time of recurrence, earlier initiation of supportive care, and reduction in routine imaging.
Source: American Society of Clinical Oncology (ASCO) Annual Meeting, June 3-7, 2016, Chicago, USA.
Literature:
- Van den Bent M, et al: Results of the interim analysis of the EORTC randomized phase III CATNON trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q co-deletion: An Intergroup trial. J Clin Oncol 2016; 34(suppl): Abs LBA2000.
- Neoptolemos JP, et al: ESPAC-4: A multicenter, international, open-label randomized controlled phase III trial of adjuvant combination chemotherapy of gemcitabine (GEM) and capecitabine (CAP) versus monotherapy gemcitabine in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol 2016; 34(suppl): Abs LBA4006.
- Venook A, et al: Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016; 34(suppl): Abs 3504.
- Venook A, et al: CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014; 32(suppl): Abs LBA3.
- Denis F, et al: Overall survival in patients with lung cancer using a web-application-guided follow-up compared to standard modalities: results of phase III randomized trial. J Clin Oncol 2016; 34(suppl): Abs LBA9006.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(5): 31-32.