Modern approaches to the treatment of inflammatory bowel disease (IBD) are based on the motto “treat-to-target”, whereby in addition to symptom relief and induction of disease remission, the aim nowadays is also to preserve and endoscopically inconspicuous colon mucosa. New agents from the field of biologics and small molecules, which can be used in cases of inadequate response to conventional drugs, are important sources of hope for optimizing therapeutic outcomes.
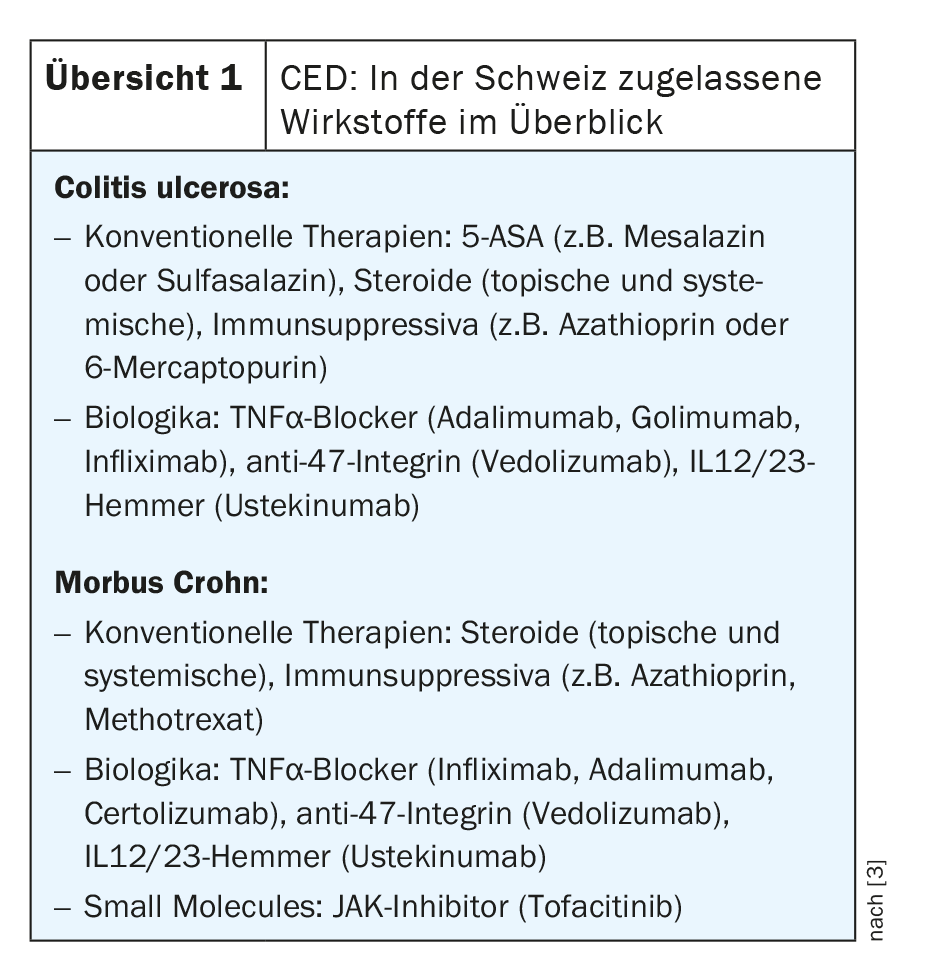
The therapeutic landscape has changed rapidly in recent years, and several new, highly effective substances from the biologics and small molecule drug groups have come onto the market, particularly for moderate and severe forms of the disease. Overview 1 shows the pharmacotherapeutic agents currently approved in Switzerland for the treatment of ulcerative colitis and Crohn’s disease.
“Treat-to-target” is the motto
The therapeutic goals in the treatment of IBD are primarily relief of symptoms and suppression of inflammation in the gastrointestinal tract. Nowadays, the aim is not only healing of the intestinal mucosa but complete control of the disease with histological remission [1]. While there is still no curative therapy, there are a variety of drug treatment options available that have been shown to be effective. In an initial phase of therapy, the focus is on induction of remission and thus symptom relief. Immunomodulatory drugs with a rapid onset of action are often used for this purpose (e.g., corticosteroids). Subsequently, it is important to maintain remission for as long a period as possible. Due to the side effects, steroids are not suitable for this purpose, but depending on the severity and other disease characteristics, aminosalicylates, azathioprine or biologics are used. In the era of personalized medicine, individual disease-specific characteristics such as risk of progression and complications as well as predictors of treatment response are increasingly taken into account [2].
For the treatment of mild ulcerative colitis (C. ulcerosa), 5-aminosalicylic acid (5-ASA) preparations are often used initially (e.g., mesalazine or sulfasalazine), and corticosteroids may also be used [3]. The focus here is on the subsiding of the inflammation. 5-ASA has also been shown to be effective as maintenance therapy and for relapse prevention. If it is a form of C. ulcerosa characterized by frequent relapses or if the disease activity is high, immunosuppressants such as azathioprine or 6-mercaptopurine may be targeted [1]. Intravenous steroids may be considered for a very active and aggressive episode of ulcerative C. ulcerosa, and if this does not provide adequate relief of symptoms, ciclosporin may be resorted to. For severe forms of progression, ustekinumab has expanded the spectrum of approved biologic therapies in Switzerland since 2020 [14].
In Crohn ‘s disease (M. Crohn), corticosteroids are usually used first if active inflammatory foci are present. In the case of mild inflammation, 5-ASA preparations may also be useful (e.g., mesalazine or sulfasalazine) [1]. In more aggressive forms of Crohn’s disease, consider the use of immunosuppressants such as azathioprine, 6-mercaptopurine, or methotrexate. If there is a lack of response to conventional drug therapy, biologics such as adalimumab, certolizumab, infliximab, vedolizumab, or ustekinumab are available.
Large arsenal of highly effective biologics
Advances in the study of molecular processes provide the basis for the development of therapeutic targets, which is reflected in new highly effective biologics. Involved is, among other things, destruction of tight junctions and the mucosal film covering the intestinal epithelial layer, which increases the permeability of the epithelium, resulting in increased uptake of luminal antigens into the deeper intestinal wall layers [23]. Involvement of immune cells results in increased production of proinflammatory cytokines – the targets of monoclonal antibodies.
The market approval of biologics has significantly improved the therapeutic options for moderate and severe forms of IBD. With the growing number of available drugs, the choice of the appropriate therapy is becoming an increasingly complex challenge. For a criterion-guided treatment decision, one can refer to a broad evidence base. Among other things, severity of symptoms and inflammatory foci in the intestine are important criteria for the treatment strategy. The following is a compact summary of selected findings on monoclonal antibodies approved today in Switzerland for the treatment of IBD.

ANTI-TNF: The approval of TNFα inhibitors a few years ago represented a major advance in the treatment options for IBD. Efficacy and safety of adalimumab for induction and maintenance therapy in moderate to severe active C. ulcerosa were demonstrated in the ULTRA 1 and 2 trials [4]. The ULTRA 3 showed that remission, mucosal healing, and improvement in quality of life were maintained with adalimumab therapy over a 4-year period [4]. The efficacy of golimumab in the presence of moderate-to-severe ulcerative colitis despite therapy with steroids, 5-ASA, or thiopurines was demonstrated in the PURSUIT trial program [5,6]. According to analyses of the PURSUIT extension study, the balance with regard to long-term data is also positive [7]. For C. ulcerosa with fulminant disease activity and poor response to intravenous steroids, the 2019 update of the S3 guidelines recommends, among others, therapy with infliximab (combined with thiopurine, if possible) [8].
Of the TNFα antagonists, certolizumab has recently been approved in Switzerland for Crohn’s disease in addition to adalimumab and infliximab. Overall, TNFα inhibitors are an important treatment option for C. ulcerosa and Crohn’s disease. However, lack of response, declining efficacy, or intolerance to anti-TNFα use was found to be a problem in some patients. For these patient groups, the anti-integrin antibody vedolizumab is an alternative [9].
ANTI-47 INTEGRIN: T cells carrying integrin-α4β7 bind to colonic endothelial cells by MAdCAM-1 (mucosal vascular addressin cell adhesion molecule 1. This results in an invasion of gut-specific T cells into the lamina propria, a layer of connective tissue underlying the epithelia [23]. Vedolizumab is an antibody directed against integrin, which has been approved in Switzerland since 2015 for moderate to severe active C. ulcerosa and Crohn’s disease in cases of inadequate response or intolerance to conventional therapy or to treatment with anti-TNFα [1]. Vedolizumab acts intestine-selectively and binds to the α4β7-integrinof additional blood cells, preventing them from triggering an inflammatory response in intestinal tissue. In a meta-analysis, rates of corticosteroid-free remission in ulcerative colitis patients were 26% at week 14 and 42% at one year [10]. In Crohn’s disease, these rates were 25% and 31%, respectively. The authors concluded that these data support the long-term benefit-risk profile of vedolizumab [10].
The VARSITY study is the first direct comparison of vedolizumab and adalimumab in patients with moderate to severe active C. ulcerosa. Vedolizumab was shown to be superior in terms of clinical remission. At week 52, 31.3% in the vedolizumab arm and 22.5% in the adalimumab arm achieved clinical remission (p=0.006) according to the Mayo score. Mucosa healing (secondary endpoint) was shown by 39.7% with vedolizumab and 27.7% with adalimumab (p<0.001) [11,12]. Furthermore, in biologic-naïve patients, infliximab and vedolizumab were found to perform best in terms of clinical remission and mucosa healing. Treatment with vedolizumab also resulted in the lowest rate of serious adverse events and infections, and the efficacy and safety profile was largely consistent with previous studies.
Table 2 shows in which cases the use of anti-integrin/vedolizumab is particularly useful.
ANTI-IL12/23: The IL12/IL23 pathway has emerged as an important factor in the pathogenesis of IBD in genome-wide association studies [13].
Ustekinumab (Stelara®) was approved in Switzerland in 2020 for the treatment of ulcerative C. Crohn’s disease , and this treatment option has been on the market since 2017 [14]. Ustekinumab is a monoclonal antibody that simultaneously blocks the proinflammatory cytokines IL12 and IL23 by targeting the p40 subunit of this hetero-dimer. In the phase III clinical trial program UNITI-1 and UNITI-2, usteki-numab was shown to be effective in both the induction phase and for maintenance therapy in patients with Crohn’s disease who had failed conventional treatment and anti-TNF therapy [15]. After a period of 6 weeks, ustekinumab showed a significantly higher clinical response (reduction of the CDAI score by at least 100 points) than the placebo condition. Study participants had each received induction therapy with ustekinumab i.v. at 2 doses (130 mg and 6 mg/kg) or placebo. Those who responded to therapy in the UNITI induction studies were enrolled in the 44-week IM-UNITI maintenance study, in which they were treated with 90 mg ustekinumab s.c. every 8 weeks, every 12 weeks, or placebo. After 44 weeks, 48.8% of patients achieved clinical remission in the group receiving ustekinumab 90 mg every 12 weeks and 53.1% in the group receiving ustekinumab 90 mg every 8 weeks. In the placebo condition, this proportion was 35.9%.
Efficacy in C. ulcerosa was demonstrated in the multicenter, double-blind, placebo-controlled phase III UNIFI trial program (Fig. 1) [15,22]. In the induction study, study participants (n=961) received a single i.v. administration of ustekinumab at a dose of 130 mg and 6 mg/kg bw, respectively, or placebo. At week 8, 15.6% of patients on 130 mg and 15.5% on 6 mg/kg versus 5.3% on placebo had achieved clinical remission (Mayo Score ≤2) [15,22]. Moreover, response rates* through week 8 were 56.5% at both doses. Clinical remission was achieved by 15.6% during this period. Patients who responded to induction therapy were included in the 44-week maintenance study (n=523). These study participants were randomly assigned to the conditions of ustekinumab 90 mg s.c. every 8 weeks (q8w), every 12 weeks (q12w), or placebo. Clinical remission at week 44 (primary endpoint) was achieved by 43.8% in the q8w arm and 38.4% in the q12w arm, compared with 24% on placebo. Remission without steroid use was even achieved in 42% (q8w) and 37.8% (q12w), respectively, at week 44; in the placebo condition, this proportion was 23.4%. In addition, calprotectin levels decreased significantly with ustekinumab (calprotectin is an important marker of inflammation in the gut) [16]. The safety profile of ustekinumab was consistent with that of previous studies.
* Reduction of ≥30% and ≥3 points in Mayo score with ≥1 point reduction in rectal bleeding subscore from baseline week 0.
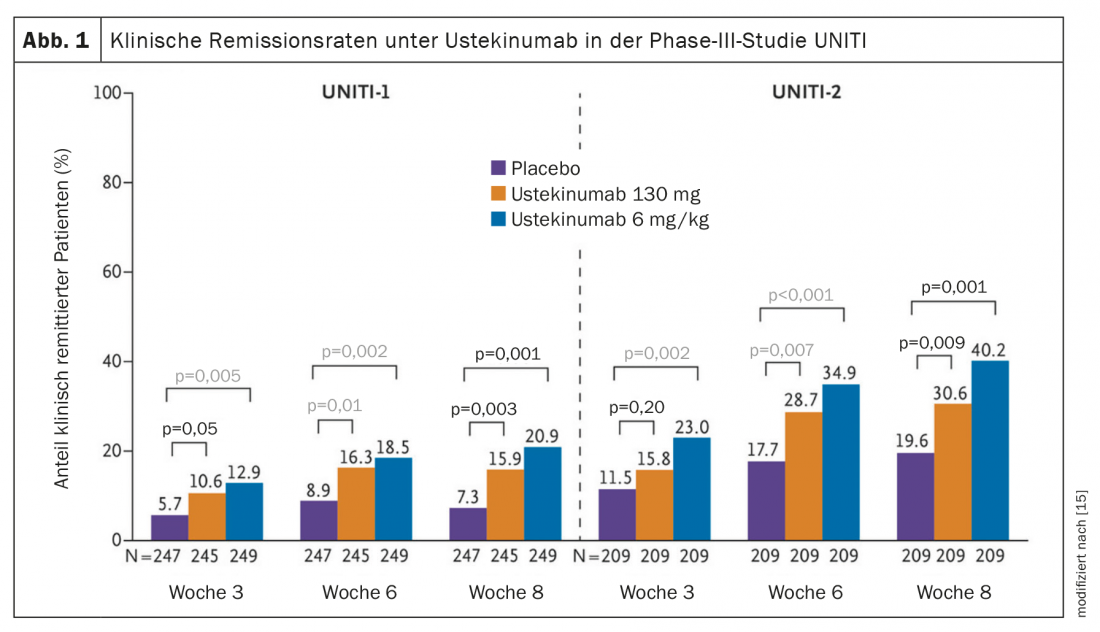
Table 3 shows in which cases the use of an IL12/23 inhibitor is particularly useful.

JAK inhibitors complement the treatment spectrum
Janus kinase (JAK) inhibitors, unlike biologics, are produced synthetically. These are low-molecular substances (“small molecules”). With regard to mechanism of action, a major difference is that JAK inhibitors, unlike biologics, do not intercept cytokine signals in the extracellular space, but intracellularly. Inhibition of the JAK-STAT pathway prevents the pro-inflammatory response by blocking multiple cytokines simultaneously. The onset of action is rapid in most cases, and the oral dosage form is an important convenience factor.
The first JAK inhibitor approved in Switzerland for CED is tofacitinib (Xeljanz®), which has been listed for the treatment of C. ulcerosa since 2019 [21]. The OCTAVE clinical trial program (“Oral Clinical Trials for Tofacitinib in ulcerative colitis global development program”) demonstrated the rapid onset of efficacy of tofacitinib [18]. After only three days, 10 mg of tofacitinib twice daily showed a statistically significantly greater decrease in rectal bleeding than placebo (14.4% vs. 8.2%; p<0.05). A significant reduction in stool frequency was detectable after seven days in a significantly higher proportion of pa-tients taking tofacitinib 10 mg twice daily compared with placebo (9.2% vs. 2.3%; p<0.01).

In the phase III U-ACHIEVE study evaluating the efficacy and safety of upadacitinib (Rinvoq®) in adults with moderate-to-severe ulcerative colitis, 26% of patients in the upadicitinib arm achieved clinical remission at 8 weeks compared to 5% in the placebo condition (p<0.001). Moreover, significantly more patients on upadacitinib experienced endoscopic improvement at week 8 compared with placebo (36% vs. 7%; p<0.001). The safety profile of utacitinib 45 mg was consistent with previous studies, with no new safety signals reported [19].
Take-Home Messages
- Modern approaches to the treatment of inflammatory bowel disease (IBD) are based on the motto “treat-to-target”, whereby in addition to symptom relief and induction of disease remission, the aim nowadays is also to preserve and endoscopically inconspicuous colon mucosa.
- Conventional anti-inflammatory drugs commonly used in IBD include aminosalicylates (5-ASA), steroids, immunosuppressants (e.g., azathioprine, 6-mercaptopurine, methotrexate, tacrolimus, cyclosporin A).
- If response is poor, biologics may be used. In addition to anti-47 integrin therapy and representatives of the TNFα blockers, an IL-12/23 inhibitor, ustekinumab, has been available for the treatment of Crohn’s disease for several years, for which an indication extension for ulcerative colitis has been granted in 2020 [14].
- From the small molecule group, tofacitinib is approved for the treatment of ulcerative colitis in Switzerland [21].


Literature:
- IBDnet: Swiss Research and Communication Network on Inflammatory Bowel Disease, www.ibdnet.ch.
- Flamant M, Roblin X: Inflammatory bowel disease: towards a personalized medicine. Therapeutic Advances in Gastroenterology 2018, https://doi.org/10.1177/1756283X17745029
- Biedermann L: State-of-the-art in IBD treatment. A general overview on conventional and advanced treatment options. PD Luc Biedermann, MD, slide presentation, December 2020 Symposium.
- Colombel J-F, et al: Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: data from ULTRA 1, 2, and 3. Am J Gastroenterol 2014; 109(11): 1771-1780.
- Sandborn WJ, et al: Subcutaneous golimumab induces clinical response and remission in patients with moderate-tosevere ulcerative colitis. Gastroenterology 2014; 146: 85-95.
- Sandborn WJ, et al: Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 96-109 e1
- Reinisch W: Long-term Benefit of Golimumab for Patients with Moderately-to-Severely Active Ulcerative Colitis: Results from the PURSUIT-Maintenance Extension. J Crohns Colitis 2018. doi: 10.1093/ecco-jcc/jjy079. [Epub ahead of print].
- Kucharzik T, et al: Updated S3 guideline ulcerative colitis. August 2019 – AWMF registry number: 021-009. www.awmf.org
- Scribano ML: Vedolizumab for inflammatory bowel disease: from randomized controlled trials to real-life evidence. World J Gastroenterol 2018; 24(23): 2457-2467.
- Schreiber S, et al: Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018; 53 (9): 1048-1064.
- National Association of Statutory Health Insurance Physicians (KBV): Biologics. Ulcerative colitis. Issue 04/2020. www.akdae.de/Arzneimitteltherapie/WA/Archiv/Biologika.pdf
- Schreiber S, et al: J Crohns Colitis 2019; 13 (Suppl 1): S612-613 (Abstract OP34).
- Jostins L, et al: Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491: 119-124.
- Specialized information Stelara®: www.swissmedicinfo.ch, last accessed 04.01.2021
- Feagan BG: Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med 2016; 375: 1946-1960.
- Sands BE, et al: Safety and efficacy of utekinumab induction therapy in patients with moderate to severe ulcerative colitis: results from the phase 3 UNIFI study. Paper presented at UEG Week 2018, Vienna, Austria.
- USZ: Clinic for Gastroenterology and Hepatology, www.gastroenterologie.usz.ch/fachwissen/morbus-crohn-colitits-ulcerosa, last accessed 04.01.2021
- Stiefelhagen P: JAK inhibition – a new promising approach. Gastro-News 5, 62 (2018). https://doi.org/10.1007/s15036-018-0464-5
- “Upadacitinib (RINVOQ™) Meets Primary and All Ranked Secondary Endpoints in First Phase 3 Induction Study in Ulcerative Colitis,” AbbVie Inc, Dec. 9, 2020.
- Stallmach A, et al: Addendum to the S3 guidelines Crohn’s disease and ulcerative colitis: care of patients with inflammatory bowel disease in the COVID-19 pandemic – open questions and answers. www.awmf.org, last accessed 04.01.2021.
- Swiss Drug Compendium: Xeljanz®, www.compendium.ch, last accessed 04.01.2021
- Ärzte Zeitung: Ustekinumab: New option for ulcerative colitis, published online Oct. 18, 2019, www.aerztezeitung.de/Medizin/Neue-Option-bei-Colitis-ulcerosa-402582.html
- Roeb E: Ulcerative colitis: therapy according to new guidelines. Pharmaceutical Journal, Feb. 14, 2019 www.pharmazeutische-zeitung.de/therapie-nach-neuer-leitlinie
HAUSARZT PRAXIS 2021; 16(1): 15-18