The range of disease-modifying therapies (DMTs) for MS patients has expanded steadily in recent years. This has also been incorporated into the new edition of the DGN guideline. The benefits and risks of the various therapeutic options must be assessed on an individual basis. Various drugs from the field of DMTs have also been newly approved in Switzerland.
Multiple sclerosis (MS) is characterized by focal, chronic inflammatory demyelinating lesions of CNS nerve fibers. These are accompanied by axonal damage, persistent tissue scarring (gliosis), and brain atrophy as the disease progresses [1]. Demyelination is thought to be initiated by different cellular and humoral factors of the innate and acquired immune system. Chronic inflammation impairs the normal transmission of nerve signals, which leads to various symptoms and can also cause permanent damage or disability in the long term. It is an incurable disease to this day. The goal of therapy is to reduce disease activity and slow progression. MS treatment is based on three pillars: relapse intervention, progression-modifying therapy, and symptom relief. Modern anti-inflammatory therapies or immunotherapies can modify the course of the disease and reduce the frequency of relapses.
What are the most important innovations of the DGN guideline?
More than a dozen compounds are now available for disease-modifying immunotherapy of MS, with different mechanisms of action, side effect spectra, and dosage forms (injection, infusion, tablets). New features include specific information on whether, when, and which of the course-modifying immunotherapies are indicated [2]. Disease-modifying drugs (DMTs) were divided into three efficacy categories – instead of the previous treatment-stage scheme. The grouping was based on the thrust rate reduction from the pivotal studies. The more mild-acting preparations also have more favorable side effect profiles. The extent of disease activity is considered an important criterion for the choice of efficacy category.
- Efficacy category 1: beta interferons, dimethyl fumarate, glatirameroids and teriflunomide.
- Efficacy category 2: cladribine, fingolimod, ozanimod
- Efficacy category 3: alemtuzumab , CD20 antibody (ocrelizumab, off label rituximab) natalizumab
Immunotherapeutic treatment is assigned to disease activity. Defined entry, change, and also exit scenarios are also presented, and special situations are addressed (e.g., pregnancy and lactation, and MS in the elderly and children/adolescents). Diagnosis was simplified by the revision of the McDonald diagnostic criteria in 2017 (CSF examination as well as MRI examination with defined sequences; extended laboratory diagnostics only in case of corresponding clinical suspicion). Diseases related to MS, such as neuromyelitis optica spectrum diseases (NMOSD) and MOG-IgG-associated diseases, were newly included as separate disease entities.
Which MS drugs are approved in Switzerland?
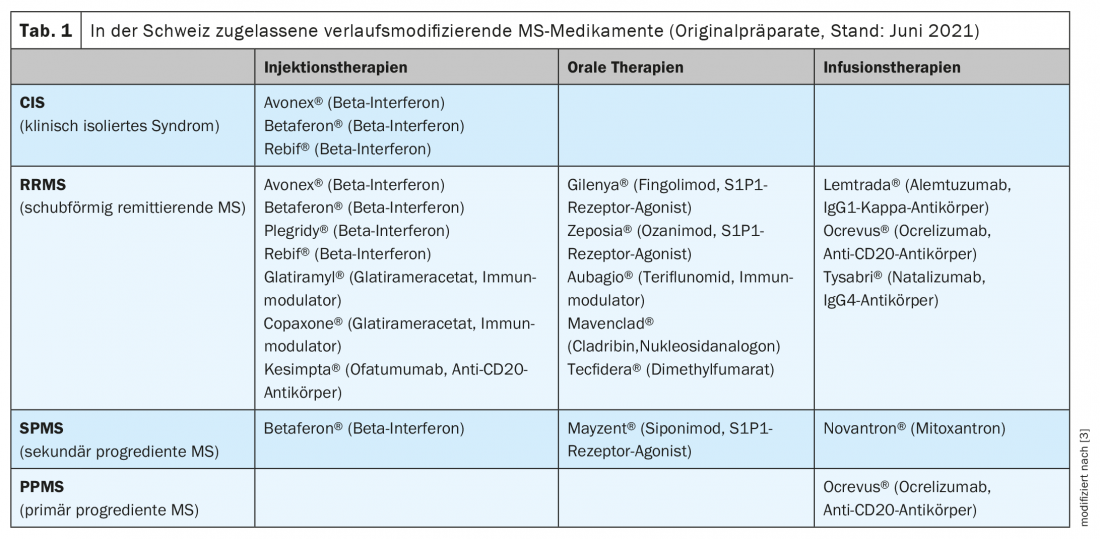
The originator drugs currently approved in Switzerland are shown in Table 1 [3,4]. For some substances, the Swissmedic approval differs in part from the approval in neighboring countries (European Medicines Agency, EMA) [5]. Furthermore, in Switzerland, limitations listed in the list of specialties by the Federal Office of Public Health (FOPH) have a significant impact on the use in clinical practice. One of the recently approved new DMTs is Kesimpta® (ofatumumab) [4]. This drug is applied under the skin once a month (pre-filled pen) after a dosing phase. Since June 1, 2021, the costs for this preparation will be covered by the basic health insurance [6].
Other recent approvals involve two members of the sphingosine 1 phosphate (S1P) receptor modulator group: Zeposia® (Ozanimod) and Mayzent® (Siponimod). While Zeposia® can be used in adults with relapsing-remitting MS (RRMS), Mayzent® is approved for individuals with secondary progressive MS (SPMS) with inflammatory disease activity (evidenced by relapses or corresponding changes on imaging)(Table 1) [3,4,7].
|
“The options available today allow for a |
Determine treatment strategy individually
Whereas escalation therapy initially involves treatment with a more moderately effective DMT and only then switching to a progression-modifying drug with a stronger effect, the principle of high-potency therapy (“highly effective therapy”) is to take advantage of an early “window of therapeutic opportunity” by using a highly potent drug at the outset. The choice of treatment strategy often represents a trade-off between high efficacy and safety/tolerability. Current evidence suggests that early intensive therapy has a medium-term beneficial effect on disability [8].
What is the mechanism of action of highly potent biologics? Recent studies on the role of B cells in disease progression have demonstrated that antibody-independent B cell function plays an important role in the pathogenesis of MS [9,10]. This is the therapeutic target of antibodies. A CD20 antibody is now available for primary progressive multiple sclerosis (PPMS), which until recently was considered untreatable. In the pivotal study of ocrelizumab, the risk of disability progression was reduced by 25% [11].
While DMTs can modify the course of the disease and reduce the frequency of relapses, acute MS relapse is still treated with cortisone in the short term. The established standard of therapy is intravenous administration of high-dose glucocorticosteroids. Treatment should be given as soon as possible after the onset of symptoms (methylprednisolone 500-1000 mg /d for 3-5 days).
The third pillar of MS therapy is non-drug interventions. Physical therapy, occupational therapy, speech therapy and other treatment options can help relieve symptoms and compensate for functional impairments.
Literature:
- Mayo L, Quintana FJ, Weiner HL: Immunol Rev 2012; 248(1): 170-187.
- Hemmer, B et al: Diagnosis and therapy of multiple sclerosis, neuromyelitis optica spectrum disorders and MOG-IgG-associated diseases, S2k-Leitlinie, 2021, in: German Society of Neurology (ed.), www.dgn.org/leitlinien, (last accessed 07/15/2021).
- Swiss Multiple Sclerosis Society, www.multiplesklerose.ch/PDF/de/Infoblaetter/01_Medizinische_und_therapeutische_Fragen/MS-Info_Behandlung_der_Multiplen_Sklerose.pdf (last accessed 07/15/2021).
- Drug Information, www.swissmedicinfo.ch, (last accessed 07/15/2021).
- Achtnichts L, et al: Specifics of immunotherapy for multiple sclerosis in Switzerland, Swiss Medical Forum 2019; 41-42.
- Swiss Multiple Sclerosis Society, www.multiplesklerose.ch/de/aktuelles/detail/kesimptar-ofatumumab-kassenzulaessig-in-der-grundversicherung (last accessed 07/15/2021).
- Swiss Multiple Sclerosis Society, www.multiplesklerose.ch/de/aktuelles/detail/neue-therapien
- Harding K, et al: Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients With Multiple Sclerosis. JAMA Neurology 2019; 76(5): 536-541.
- Fraussen J, et al: B cells and antibodies in progressive multiple sclerosis: Contribution to neurodegeneration and progression. Autoimmune Rev 2016; 15: 896-899.
- Jelcic I, et al: Memory B Cells Activate Brain-Homing, Autoreactive CD4+ T Cells in Multiple Sclerosis. Cell 2018; 175: 85-100.
- Montalban X, et al; ORATORIO Clinical Investigators: Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 2017; 376(3): 209-220.
- DGN: “New S2k Guideline for Diagnosis and Therapy of Multiple Sclerosis”, German Society of Neurology, 10.05.2021.
HAUSARZT PRAXIS 2021; 16(8): 26-27