There has been a groundbreaking increase in knowledge in hemostaseology in recent years, both preclinically in basic research and clinically with the development of innovative treatment strategies. Using the patient as a benchmark means highlighting the latest developments in hemostaseology research in terms of their impact on current and future patient care. This year’s annual conference lived up to this claim.
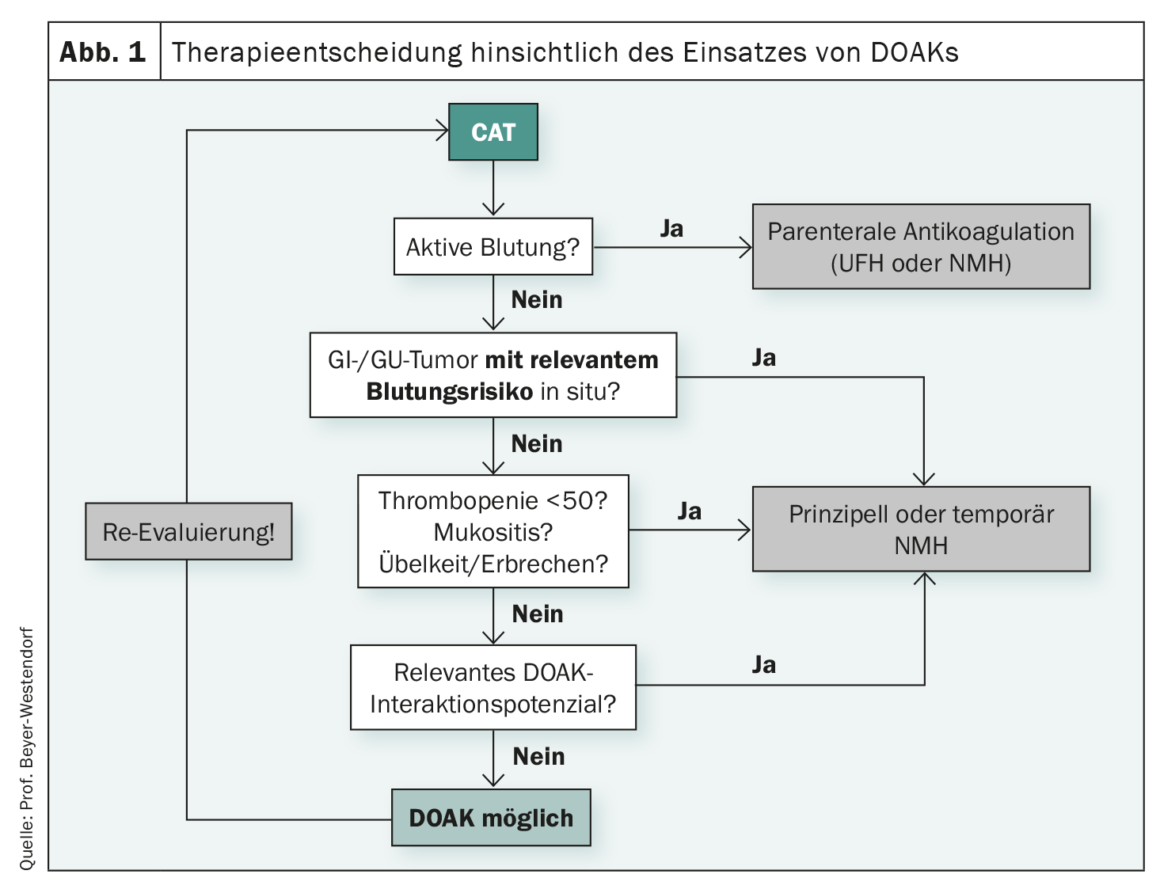
Guidelines are for simplifying decisions in everyday practice. However, this is not always the case, as Prof. Dr. med. Jan Beyer-Westendorf, Dresden (D), demonstrated using anticoagulation in tumor-associated venous thromboembolism (tumor VTE). When anticoagulation is to be initiated in tumor patients, it is known that the risk of recurrence as well as bleeding is very high. With vitamin K antagonist (VKA) therapy, recurrent VTE was observed in 6.8% of nononcology patients and 20.7% of cancer patients. The risk of major bleeding is 12.4% for tumor patients receiving VKA therapy and 4.9% for patients without oncologic disease. Complicating matters further, the risk of VTE continues to change over time. Accordingly, the risk-benefit trade-off for anticoagulation in cancer associated thromboembolism(CAT) patients is particularly difficult, the expert emphasized. If one now consults the guidelines, the current recommendations are that DOAK and NMH should be used for the therapy of tumor VTE. This reflects a paradigm shift, because it is no longer a question of whether to anticoagulate at all, unfractionated heparin and vitamin K antagonists play a role only very rarely, and the therapeutic standard of the last 20 years with low-molecular-weight heparins (NMHs) is now supplemented by direct oral anticoagulants (DOAKs). The real key question today is rather when to resort to NMH and when it is better to resort to DOAK. A clue may be provided by the patient selection of the DOAC-CAT studies. Here, thrombopaenic patients, sufferers with brain tumors, and sufferers with upper GI tract tumors were generally excluded or underrepresented. In addition, there is an increased risk of bleeding with DOAKs and the risk of bleeding varies depending on tumor type. The potential for interaction with other drugs should also be considered, he said. In his experience, DOAK therapy reaches its clinical limits in patients with dysphagia, mucosal lesions, and nausea or vomiting and when anticoagulation is required despite bleeding stigmata and clinically relevant thrombocytopenia (Fig. 1). In principle, however, Beyer-Westendorf is convinced that NMH and DOAK are equivalent and important “side-by-side” options for tumor patients with VTE, and that many affected individuals can also benefit from the simplicity of DOAK use, but that close attention should be paid to when, among other things, contraindications complicate or even prevent their use.
Women-specific aspects in CAT
The incidence of VTE in tumor disease is high – especially in ovarian cancer. In addition, the incidence is stage-dependent. While pancreatic carcinoma still ranks first and ovarian carcinoma second in stages I and II, these are overtaken by endometrial carcinoma in stage IV. If one directs one’s attention to the effects of VTE on mortality, these show clear effects in endometrial and breast carcinoma, as Christian Pfrepper, MD, Leipzig (D), explained. Accordingly, thrombosis prophylaxis plays a completely different role in gynecological tumors. This should be performed during chemotherapy in high-risk patients with apixaban, rivaroxaban, or NMH. For tumor surgery in the abdomen/pelvis, NMH, UFH, or apixaban should be administered for four weeks unless there is a high risk of bleeding.
Sandra Marten, MD, Dresden (D), added that the main indication for anticoagulation in patients younger than 50 years is prevention and treatment of VTE. DOAKs are largely used for this purpose – increasingly also in women of reproductive age. This presents new challenges regarding the effect of therapy on menstrual bleeding, prevention or planning of pregnancy, and the risk of DOAK exposure during pregnancy and lactation. There are many patient discussions on the internet around the topic of increased menstrual bleeding under DOAK. This is also shown by the large studies in which hypermenorrhea or prolonged menstruation was observed. Only dabigatran did not show this signal. This is partly because of the altered hormonal status due to changes in contraception or hormone therapy after VTE, and partly because of the direct effect of DOAKs on clotting factors, the uterine wall, and synergism with natural anticoagulants required for menstrual bleeding, she said. If hypermenorrhea or atypical uterine bleeding occurs, the DOAK dose should be reduced or switched to dabigatran, or treatment should be stopped and switched to ASA.
The next question relates to avoiding or planning pregnancy while on DOAK therapy. Adequate contraception is undoubtedly the right approach, the expert said. The increase in risk for recurrent VTE with hormonal contraceptives seems small, and the benefit is superior to the risk. Before de-escalation of DOAK therapy, re-evaluation including a switch from the estrogen-containing pill to a safer alternative is advised.
With regard to planned pregnancy, consider switching to a different anticoagulant. Switching to NMH is safe, effective, and there are no signs of embryotoxicity. However, the treatment is unpleasant and associated with risks such as HIT and osteoporosis. Switching to VKA is possible until pregnancy occurs due to known embryotoxicity. Oral therapy is otherwise effective and valid data and recommendations are available. The critical exposure period at gestational week six to twelve and the frequent overlap due to detection of pregnancy and the half-life of the VKA should not be underestimated. In principle, the patient could also remain on DOAK therapy until pregnancy is confirmed. Treatment is also oral, effective, and DOAKs have a very short half-life siezti. However, the unknown embryotoxicity risk and the lack of recommendations to date speak against this, Marten summarized her review.
During pregnancy, on the other hand, the switch from DOAKs to NMH should be made in any case. Nevertheless, DOAK exposure is not a reason to terminate pregnancy. However, early gynecologic co-management and fetal monitoring are recommended. DOAK therapy during breastfeeding is also not indicated. Therefore, depending on the risk-benefit balance, either breastfeeding should be discontinued or switched to NMH.
Congress: 67. Jahrestagung der Gesellschaft für Thrombose- und Hämostaseforschung (GTH) 2023
InFo ONKOLOGIE & HÄMATOLOGIE 2023; 11(2): 20–21