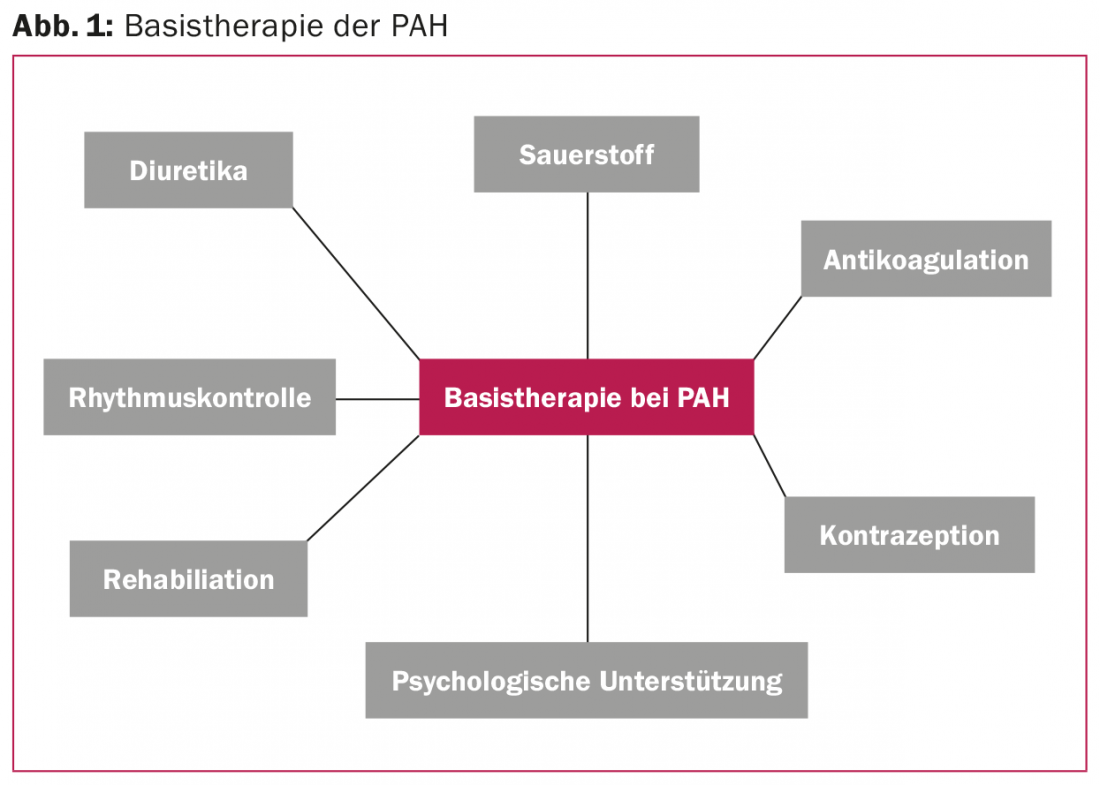
Treatment of pulmonary arterial hypertension (PAH) includes nonspecific measures (basic therapy) and specific drugs (selective pulmonary vasodilators). An extended basic therapy, including oral anticoagulation, rhythm control, rehabilitation measures and psychosocial support in case of depressive symptoms, is essential. Specific vasodilators reduce morbidity and mortality of PAH. Currently, several compounds are available that affect one of the three PAH signaling pathways: Endothelin, NO, or prostacyclin cascade. Substances that affect the same signaling pathway (e.g., riociguat and sildenafil or macitentan and bosentan) must not be combined.
Pulmonary arterial hypertension (PAH) is a disease of the peripheral pulmonary vasculature that, if left untreated, leads to a progressive increase in pulmonary vascular resistance and a reduction in the size of the pulmonary circulation. The prognosis is determined by the ability of the right ventricle to tolerate this increased afterload.
About 30 years ago, the median survival after the diagnosis of “primary pulmonary hypertension” was 2.8 years [1]. Since the first therapeutic approaches with calcium antagonists and oral anticoagulation, the therapeutic options have multiplied. Whereas initially the focus was on nonspecific measures such as treatment of right heart failure with diuretics and digoxin, drugs have since been developed that selectively influence various signaling pathways of pulmonary vasoconstriction and inhibit disease progression. Ten compounds are now approved for the treatment of PAH [2]. However, in addition to this selective pulmonary vasodilation, individualized basic therapy has not lost its importance and should complement any specific PAH therapy (Fig. 1).
Selective pulmonary vasodilators.
Endothelial dysfunction and the resulting imbalance of vasoconstrictors versus vasodilators is an important aspect in the pathogenesis of PAH. Endothelial dysfunction affects vascular remodeling of the pulmonary circulation as well as hemostasis and coagulation, which in turn contribute to the progression of PAH. The PAH drugs used today target one of the three known signaling pathways that influence pulmonary pressure: Prostacyclin (PGI2) [3], nitrogen (NO) [4], and the endothelin-1 pathway [5] (Fig. 2).
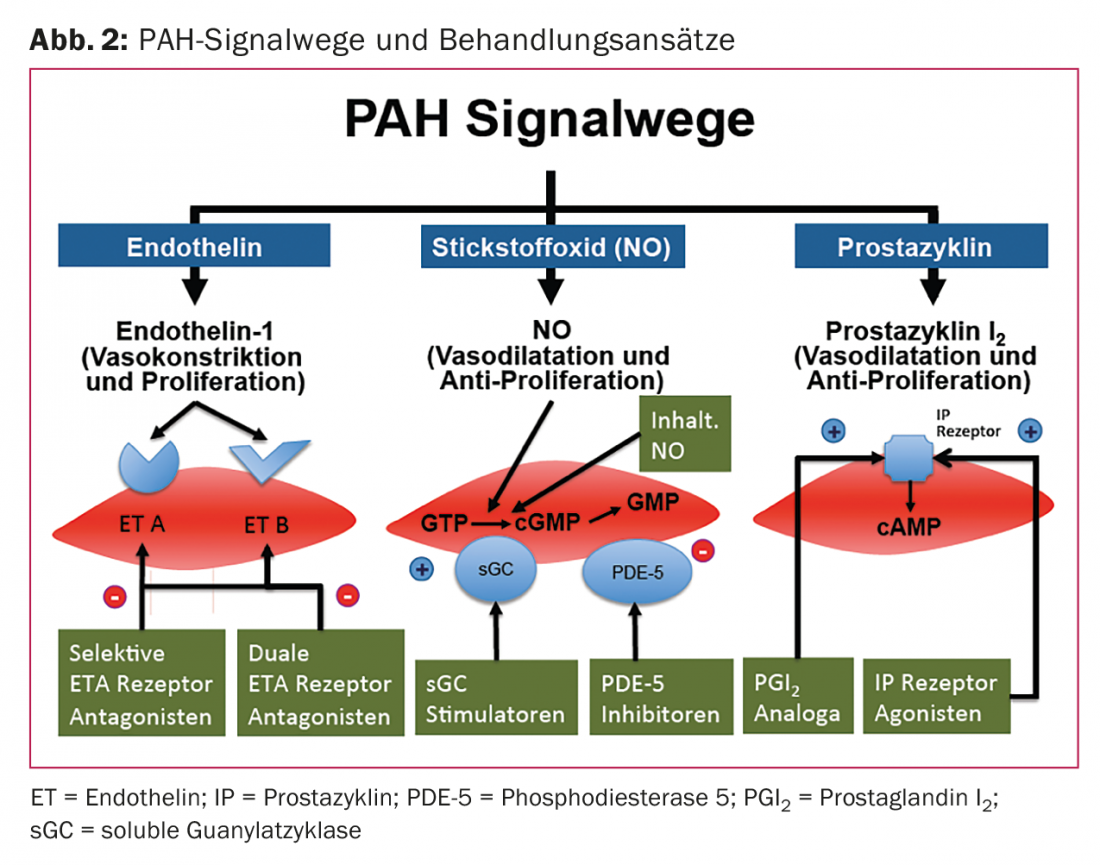
PAH is characterized by upregulation of vasoconstrictors (eg, endothelin-1) and reduction of vasodilators (eg, PGI2 and NO).
Since 2004, pulmonary vasodilators from all three pathways have been available to us. In recent years, drugs have been tested that are either advancements of existing molecules (e.g., macitentan), target new points in the signaling cascade (e.g., riociguat), or include a novel molecule that can bind to identical receptors as existing compounds (e.g., selexipag). It is hoped that these new agents will have improved pharmacokinetic properties that will simplify administration, increase efficacy and/or be associated with a lower side effect profile.
Macitentan
Macitentan (Opsumit®) is the latest addition to the group of endothelin receptor antagonists. Other endothelin receptor antagonists approved in Switzerland are bosentan (Tracleer®) and ambrisentan (Volibris®). Endothelin-1 has marked vasoconstrictive potential and leukocyte-stimulating effects. Binding to the G protein-coupled endothelin receptor isoforms A (ETA) and B (ETB) of smooth muscle cells mediates pulmonary artery vasoconstriction. Like bosentan, macitentan acts via dual receptor blockade. Due to longer active metabolites and delayed receptor dissociation, macitentan is postulated to have a better pressure-lowering effect [6].
The efficacy of macitentan was evaluated in a phase III trial (SERAPHIN trial) in 742 patients [7]. They received placebo, macitentan 3 mg/day, or macitentan 10 mg/day in a 1:1:1 ratio. Additionally, therapy with prostanoids or phosphodiesterase inhibitors could be used. SERAPHIN is the largest double-blind, randomized placebo-controlled, long-term study in patients with PAH to date and features an innovative study approach as an event-driven outcome study. Using these data, macitentan was shown to reduce the combined end point of morbidity and/or mortality by 45% in patients with PAH compared with placebo.
Figure 3 shows Kaplan-Meier graphs of the long-term effect in terms of morbidity and mortality in patients with and without combination therapy with other pulmonary vasodilators.
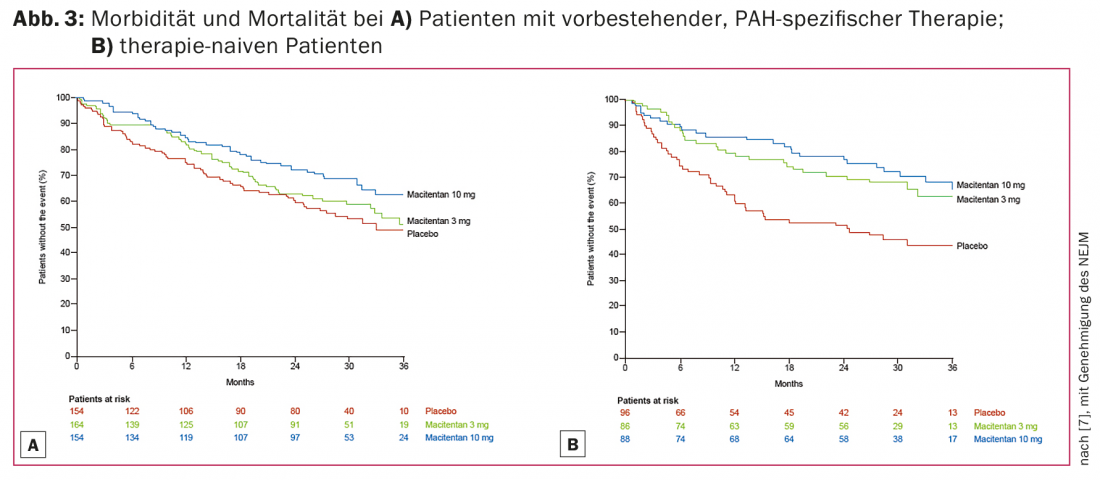
Based on these trial data, macitentan 10 mg daily has been approved since February 2014 for the treatment of PAH with a functional class II-III as defined by WHO. Compared to the established bosentan, it induces fewer drug-drug interactions and has fewer side effects; in particular, transaminases do not need to be checked monthly. The most common side effects are nasopharyngitis, anemia, and headache.
Macitentan is primarily degraded in the liver via cytochrome P450 3A4 (CYP3A4). It must therefore be noted that inducers of CYP3A4 (St. John’s wort, anticonvulsants, rifampicin, etc.) and inhibitors of CYP3A4 (grapefruit juice, macrolide antibiotics, metronidazole, antifungals, HAART, etc.) can lead to altered drug levels.
Riociguat
Riociguat belongs to a new class of drugs PAH therapy. Like conventional phosphodiesterase-5 (PDE-5) inhibitors, it acts via the vasodilating nitric oxide (NO) signaling pathway. Riociguat stimulates guanylate cyclase independently of NO, resulting in an increase in intracellular cyclic guanosine monophosphate (cGMP). cGMP, in turn, influences calcium concentration and thus pulmonary artery smooth muscle cell contractility. The conventional PDE-5 inhibitors inhibit the degradation of cGMP. Their action thus requires basal, NO-mediated cGMP production. Because PAH is associated with decreased endogenous NO production, this may limit the efficacy profile of PDE-5 inhibitors. This theoretical disadvantage is circumvented with riociguat.
Large, placebo-controlled trials have demonstrated the efficacy of riociguat in improving walking distance in the 6-minute walk test for patients with PAH (PATENT trial) [8] and for patients with inoperable or persistent/recurrent chronic thromboembolic pulmonary hypertension after thromboendarterectomy (CHEST trial) [9]. Both phase III studies confirmed a good safety profile, with pulmonary hemorrhage occurring in a few cases.
The dosage of riociguat should be individualized; the maximum dose is 2.5 mg three times daily. The most common side effects are hypotension, headache, dizziness, and gastrointestinal discomfort including. Dyspepsia. Combination with PDE-5 inhibitors or NO preparations is contraindicated, and combinations with antiplatelet agents should be well evaluated on a case-by-case basis.
Selexipag
Selexipag is the first prostaglandin I2 receptor agonist available in tablet form that also does not consist of a prostacyclin molecule. Until now, only prostacyclin analogues could be used, which had to be administered continuously intravenously or subcutaneously or inhaled every three hours due to a very short half-life.
The prostacyclin pathway has a special role in the treatment of PAH in that only epoprostenol (prostaglandin I2 analog for continuous intravenous administration) has been documented to directly reduce mortality in a placebo-controlled study [10]. Continuously administered prostacyclins are the therapy of choice for decompensated PAH.
The effect of selexipag was first evaluated in a small phase II study of 43 patients already on PAH therapy with endothelin receptor antagonists or PDE-5 inhibitors (or both) [11]. Under selexipag, a reduction in pulmonary resistance of >30% was achieved, regardless of whether patients were pretreated or not.
In the phase III Griphon study with over 1100 patients and a combined clinical endpoint (mortality and morbidity), the benefit of selexipag was again shown to be independent of gender, pre-existing medication, severity of performance impairment, age and cause of PAH. The primary endpoint was met by 42% of patients on placebo vs. 27% of patients on selexipag after an average of 70 weeks [12].
Assuming that the density of prostacyclin receptors is variable from patient to patient and, consequently, that individually different doses are required for maximal blockade, an up-titration of the selexipag dose was provided (400-3200 µg/d). When side effects (headache, diarrhea, nausea, or jaw pain) occurred, the next lower dose was selected as the maintenance dose. Since no difference was observed between patients receiving high and low doses in the endpoint analysis, this approach is considered to be valid (Fig. 4).
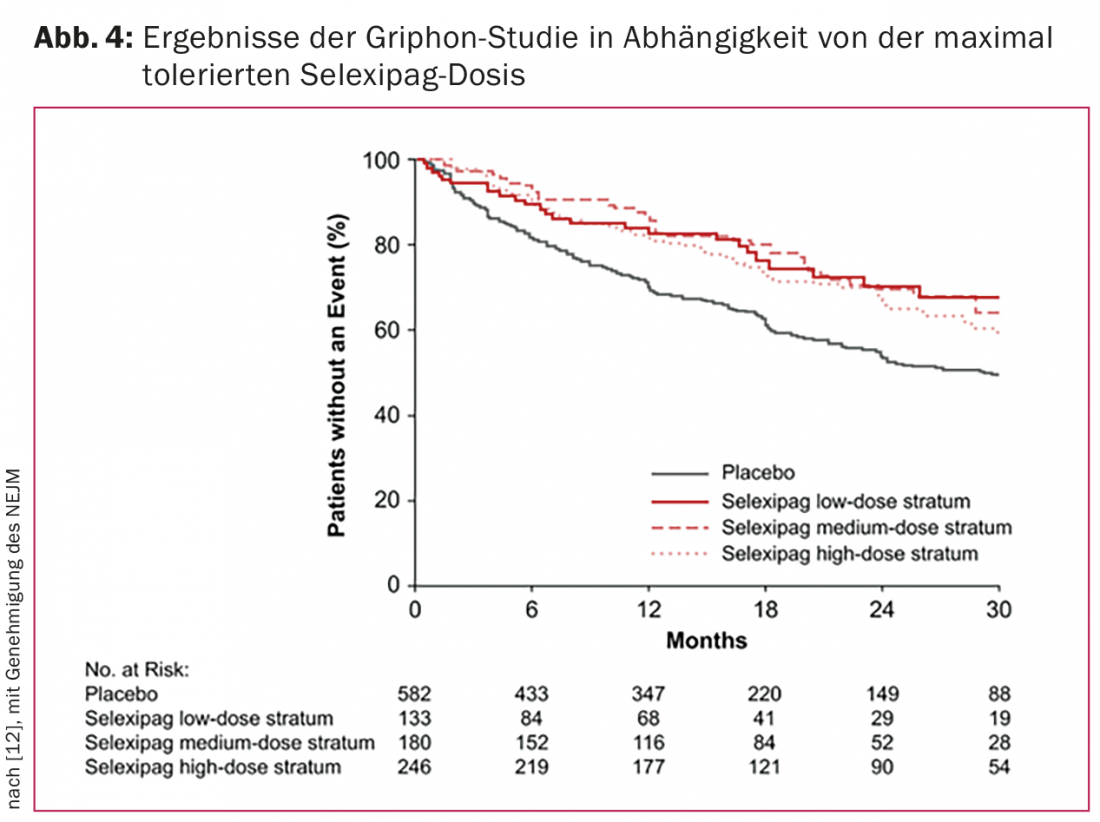
Selexipag is approved in Switzerland as of mid-2016. Similar to the drug riociguat, it will be another PAH drug that will not be prescribed in a fixed dose but will need to be individually titrated. Like all other agents, it should be prescribed only by physicians experienced in PAH treatment, ideally through established PAH centers.
Literature:
- Rich S, et al: Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987; 107(2): 216-223.
- Humbert M, et al: Advances in Therapeutic Interventions for Patients With Pulmonary Arterial Hypertension. Circulation 2014; 130(24): 2189-2208.
- Giaid A, Saleh D: Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995; 333(4): 214-221.
- Christman BW, et al: An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992; 327(2): 70-75.
- Giaid A, et al: Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993; 328(24): 1732-1739.
- Iglarz M, et al: Comparison of pharmacological activity of macitentan and bosentan in preclinical models of systemic and pulmonary hypertension. Life Sci 2014; 118(2): 333-339.
- Pulido T, et al: Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369(9): 809-818.
- Ghofrani HA, et al: Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369(4): 330-340.
- Ghofrani HA, et al: Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369(4): 319-329.
- Barst RJ, et al: A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 334(5): 296-302.
- Simonneau G, et al: Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur Respir J 2012; 40(4): 874-880.
- Sitbon O, et al: Selexipag for the Treatment of Pulmonary Arterial Hypertension. N Engl J Med 2015; 373(26): 2522-2533.
CARDIOVASC 2016; 15(2): 6-10