The prognosis of light chain amyloidosis (AL), a plasma cell dyscrasia, is largely determined by the presence of cardiac involvement. Conventional staging is based on cardiac biomarkers and free light chain difference. A new study now sought to evaluate the role of echocardiographic parameters as prognostic markers in AL amyloidosis and to investigate their utility compared with conventional staging.
Cardiac amyloidosis is the archetype of infiltrative cardiomyopathy and often presents with the clinical phenotype of heart failure with preserved ejection fraction. Systemic light chain amyloidosis (AL amyloidosis) is a plasma cell dyscrasia with multisystem involvement; however, the prognosis of AL amyloidosis is often related to cardiac involvement. This is reflected in the use of the “Mayo score” in staging AL amyloidosis. This score includes the cardiac biomarkers high-sensitivity troponin (hs-Trop) and N-terminal pro-brain natriuretic peptide (NT-proBNP), as well as the difference in free light chains (dFLC) [2].
The original and revised Mayo scores for grading AL amyloidosis have demonstrated prognostic utility, but there are limitations to the use of these biomarkers. The differences in troponin assays used at different centers, eg, hs-TropI and hs-TropT, each of which has its own defined ranges, make temporal comparisons difficult. Tests for NT-proBNP have also changed over time, representing similar implications to troponin. In addition, both troponin and NT-proBNP are influenced by factors such as body mass index and coexisting renal insufficiency, the latter being relatively common in systemic AL amyloidosis.
In contrast, traditional echocardiographic markers of cardiac structure and function are standardized and have universally defined normal ranges. In addition, transthoracic echocardiogram (TTE) is routinely performed in patients with AL amyloidosis to assess cardiac involvement because it is inexpensive and widely available. New echocardiographic indices such as left ventricular global longitudinal strain (LVGLS) have been validated for a wide range of disease processes and are increasingly used in the clinical evaluation of AL amyloidosis [3]. Therefore, a recent study investigated the prognostic utility of echocardiographic parameters compared with Mayo staging in patients with AL amyloidosis [1].
Patient characteristics and echocardiographic parameters
Seventy-five consecutive patients with AL amyloidosis who were evaluated at a referring amyloid clinic and underwent a comprehensive echocardiographic examination were retrospectively identified. The mean age was 61.8 ± 10 years, and 51/75 patients were male. 71 of 75 patients received chemotherapy, most of them with bortezomib (Velcade), cyclophosphamide, and dexamethasone (VCD; 30/75); 11 patients underwent allogeneic stem cell transplantation. Twenty-five patients were classified as Mayo stage I, 22 as stage II, while stages III and IV included 15 and 13 patients, respectively.
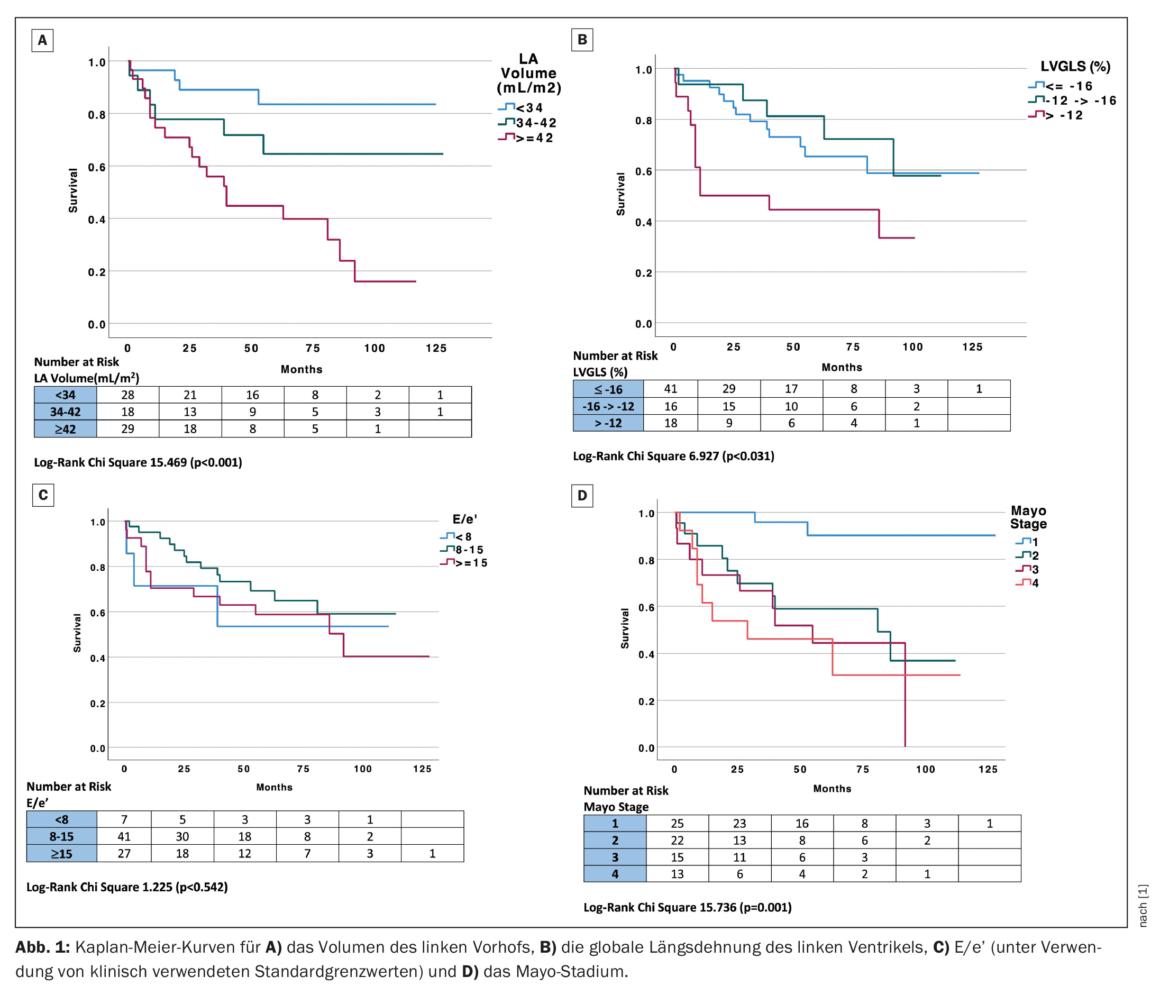
Echocardiographic parameters studied included left ventricular ejection fraction, mass, diastolic function parameters, global longitudinal strain (GLS), and left atrial (LA) volume. Mortality was determined by a review of clinical records. During a median follow-up of 51 months, 29/75 (39%) patients died. Patients were divided into two subgroups based on mortality, which did not differ in age, sex, or body mass index, although systolic blood pressure reached statistical significance (p=0.051). 11 patients had AF; the rate of AF was similar in both groups: 6/46 (13%) in survivors vs. 5/29 (17%) in non-survivors (p=0.617). Patients who died had significantly greater LA volume (35 ± 10 vs. 47 ± 12 mL/m2, p<0.001) and higher E/e’ (14 ± 6 vs. 18 ± 10, p=0.026); both LV mass and LVGLS approached statistical significance. There were no differences between groups in LV systolic function assessed by LVEF. As expected, mortality increased with increasing Mayo stage and was 8, 45, 60, and 62%, respectively, in patients in Mayo stages I-IV.
Mayo stage and univariate echocardiographic predictors of survival.
Univariate analysis revealed that the echocardiographic parameters that predicted mortality with a significance of p<0.1 included LAVI, e’ velocity, E/e’, and LVGLS. Mayo stage was also an independent predictor of survival. Univariate echocardiographic predictors of mortality (significance of p<0.1) were entered into a multivariable Cox proportional hazards model and included E/e’ and LA volume and LVGLS (e’ velocity was not included in the model because of collinearity with E/e’). In this multivariable model of echocardiographic parameters, LAVI was the only independent echocardiographic predictor of mortality. Indexed left atrial volume correlated with Mayo stage (Spearman correlation coefficient 0.5, p<0.001), and all patients in Mayo stages III and IV had dilated left atria (LAVI ≥34 mL/m2).
Classification of patients into three different risk groups
LVGLS (left ventricular systolic function), LAVI, and E/e’ (diastolic function) were analyzed as categorical variables using clinically applicable cut-off values to assess their impact on mortality compared with Mayo stage. Global longitudinal left ventricular strain was divided into three groups based on the previously reported clinical cutoff of better than -16, -12 to -16%, and worse than -12% for normal, reduced, and severely reduced GLS, respectively [4]. Indexed left atrial volume was divided into three groups using a clinical cutoff of ≤34, 34-42, and ≥42 mL/m2, corresponding to normal, mild to moderate LAVI dilation, or greater than moderate LAVI dilation [5]. ‘E/e’ was divided into three groups based on clinical values of <8, 8-15, and ≥15, corresponding to normal, probably abnormal, and elevated LV filling pressure, respectively [6]. Stratification by clinical group of LAVI (p<0.001), LVGLS (p=0.031), and Mayo stage (p=0.001) were significant predictors of mortality, while E/e’ did not reach significance in Kaplan-Meier analysis (Fig. 1) [1]. Of note, patients with a normal LAVI <34 mL/m2 had particularly good long-term outcomes (median follow-up 60.5 ± 46 months) and, conversely, patients with an LVGLS of less than -12% had a poor outcome.
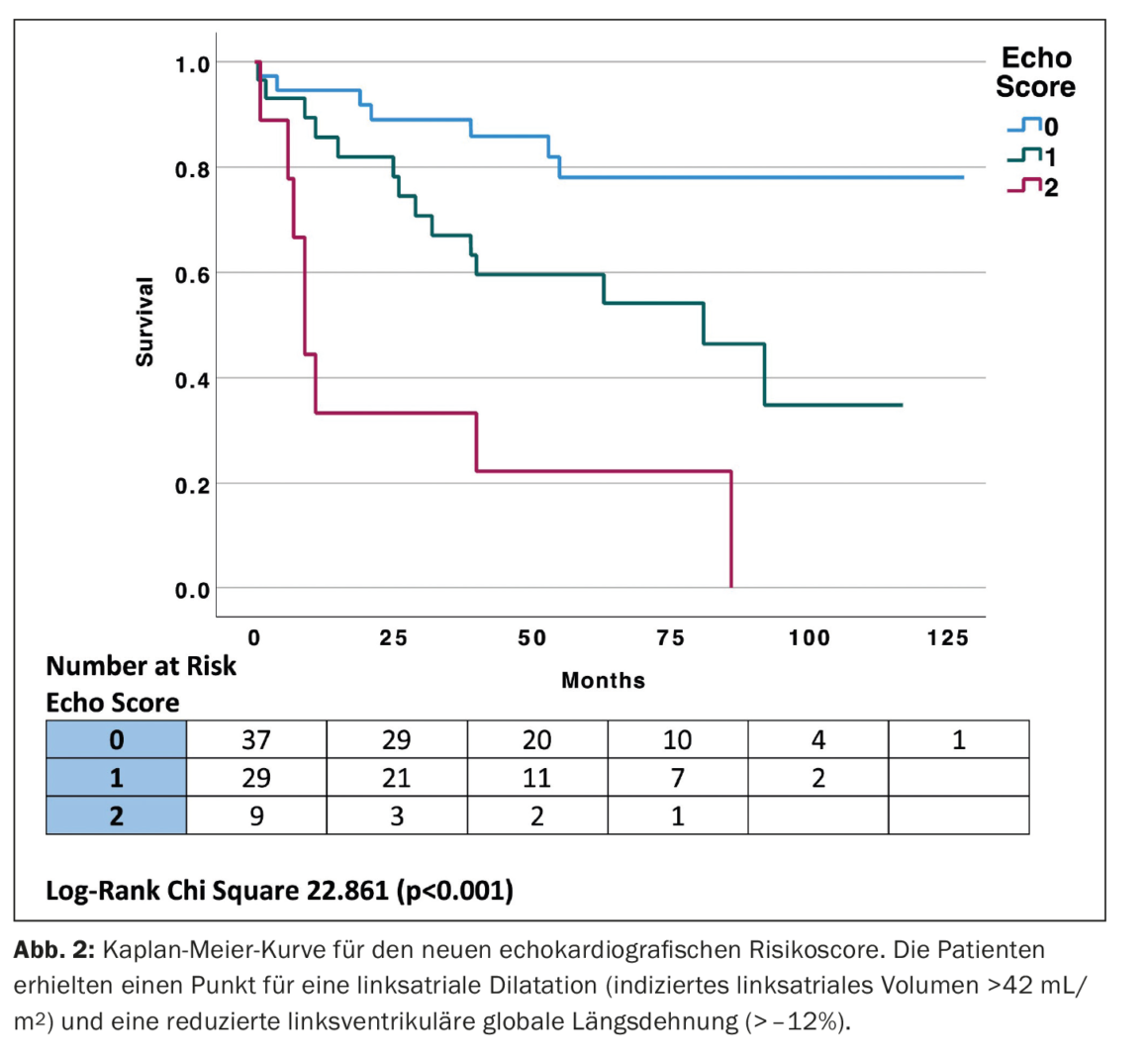
Scoring system for left atrial dilatation and reduced left ventricular global longitudinal strain.
Using the clinical cutoffs for LVGLS and LAVI to generate a simple echocardiographic score, the highest-risk group showed both LVGLS (worse than -12%) and LAVI (>42 mL/m2), the intermediate-risk group had LVGLS -12 to -16% and LAVI 34-42 mL/m2, and the lowest-risk group had LVGLS better than -16% or LAVI <34 mL/m2. Subsequently, a risk score was established by assigning one point each for LVGLS worse than -12% and LAVI >34 mL/m2, placing patients into one of three groups. The highest-risk group had both LVGLS (worse than -12%) and LAVI (>42 mL/m2), the intermediate-risk group had either LVGLS worse than -12% or LAVI >42 mL/m2 and the lowest risk group had LVGLS better than -12% and LAVI <42 mL/m2 with two, one, and zero points, respectively. Kaplan-Meier curves for the new echocardiographic risk score (Echo score) (Fig. 2) [1] were constructed. The new “echo score” had similar prognostic performance to Mayo stage [AUC 0.745, 95% confidence interval (CI) 0.638-0.853 vs. AUC 0.752, 95% CI 0.645-0.858, p=0.911].
Prediction of all-cause mortality comparable with existing Mayo staging system
The intra- and interobserver variability of LAVI was excellent, with an intraclass correlation coefficient of 0.987 (95% CI 0.946-0.997), whereas the interobserver correlation coefficient was 0.935 (95% CI 0.731-0.984). LVGLS was also highly reproducible with an intraclass correlation coefficient of 0.989 (95% CI 0.864-0.998), while the interobserver correlation coefficient was 0.980 (95% CI 0.924-0.995). Left ventricular ejection fraction showed good within- and between-observer variability, with an intraclass correlation coefficient of 0.871 (95% CI 0.517-0.967) and an interobserver coefficient of 0.772 (95% CI 0.115-0.943). These results demonstrate that cardiac imaging can be used to identify patients with higher mortality, so such risk stratification may potentially lead to the selection of optimal therapies.
Take-Home Messages
- A simple echocardiographic parameter, LAVI, was an independent prognostic marker in patients with AL amyloidosis.
- Indexed left atrial volume and LVGLS stratified by clinical cut-off values showed worse outcomes with worse LVGLS and increasing LAVI.
- A composite echocardiographic score derived from LAVI and LVGLS has similar predictive value to Mayo staging.
Literature:
- Genty P, et al.: A novel echocardiographic risk score for light-chain amyloidosis. Eur Heart J 2023; https://doi.org/10.1093/ehjopen/oead040.
- Kumar S, et al.: Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012; 30: 989–995.
- Cohen OC, Ismael A, Pawarova B, et al.: Longitudinal strain is an independent predictor of survival and response to therapy in patients with systemic AL amyloidosis. Eur Heart J 2021; 43: 333–341.
- Potter E, Marwick TH: Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 2018; 11: 260–274.
- Lang RM, et al.: Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14.
- Ommen SR, et al.: Clinical utility of Doppler echocardio-graphy and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 2000; 102: 1788–1794.
CARDIOVASC 2023; 22(2): 50–52
| Cover image: High magnification micrograph of senile cardiac amyloidosis. Congo red stain. Autopsy specimen. ©Wikimedia (Nephron) |