Targeted treatment approaches have added valuable options to the therapeutic landscape in cancer. Promising results on targeted therapy of advanced ovarian and endometrial cancer were presented at this year’s ESMO and ASCO annual congresses.
For patients with advanced ovarian cancer (OC) and homologous recombination deficiency (HRD) who responded to first-line platinum-based chemotherapy (1L-CT), beginning in October 2021, the cost of therapy for first-line maintenance therapy with the Poly ADP ribose polymerase inhibitor (PARPi) Niraparib
(product name: Zejula)
Covered by health insurers [1, 2]*. The primary analysis of the phase III PRIMA trial was pivotal, in which niraparib more than doubled median progression-free survival (PFS) in HRD-positive OC patients and reduced the risk of progression or death by 57 % after a median follow-up of 1.2 years [3]. PFS was assessed by an independent, central, blinded committee and was consistent with the investigator’s assessment [3].
Niraparib: significant PFS benefit even after 3.5 years [4]
According to an update of the PRIMA trial presented at this year’s European Society for Medical Oncology (ESMO) Annual Congress, niraparib maintained its PFS benefit over the long term. Thus, according to the investigator’s assessment, mPFS was more than doubled (24.5 months versus 11.2 months) with niraparib compared with placebo in HRD-positive OC patients, even after a median follow-up of 3.5 years, and the risk of progression or death was reduced by 48 % reduced (95 % CI: 0.40 – 0.68; p<0.001) [4].
The greatest treatment success was achieved in patients with HRD-positive, BRCA-mutated (BRCAmut) disease (HR: 0.45; 95 % CI: 0.32 – 0.64). However, even in the subgroup of HRD-positive BRCAwt (HR: 0.66; 95 % CI: 0.44 – 1.00) OC patients, niraparib was superior to placebo in terms of PFS [4].
The rate of treatment discontinuation with niraparib was also low in long-term follow-up, at 14 %. The tolerability profile was consistent with the primary analysis of the study and no new safety signals emerged [4]. Thus, niraparib is currently the only first-line mono-maintenance PARPi therapy approved in Switzerland with a proven PFS benefit in HRD-positive OC patients also in the long term [2, 4].
Niraparib versus active surveillance associated with prolonged real-world PFS [5]
That PARPi can also prolong PFS under real-world conditions is supported by a real-world analysis presented at this year’s ASCO annual meeting [5]. This evaluated the electronic dossiers of patients enrolled in the U.S. Flatiron Health network who received PARPi monotherapy or were actively monitored after platinum-based 1L CT [5]. Real-world PFS was calculated using Kaplan-Meier methods and Cox models and was defined as the time from the end of 1L CT to the next therapy, the occurrence of death, or the end of the study period. Of the total 705 patients, 166 (23.5%) received PARPi monotherapy with niraparib (n=65), olaparib (n=89), or rucaparib (n=12). 539 (76.5%) patients were actively monitored. In patients with BRCAmut OC, mPFS was also not achieved with PARPi monotherapy and was 11.4 months with active surveillance. According to multivariate analysis, 1L PARPi maintenance therapy was an independent predictor of longer PFS compared with active surveillance, once again suggesting the use of PARPi as first-line maintenance therapy.
Dostarlimab: durable response in endometrial cancer [6]
The anti-PD-1 antibody dostarlimab (Product name: Jemperli) has been approved in Switzerland since February 2022 as the first immunotherapy for the treatment of patients with recurrent or advanced endometrial cancer (EC) and defective DNA mismatch repair (dMMR)/high microsatellite instability (MSI-H) that was progressive during or after platinum-based chemotherapy [7, 8]. The approval is based on data from the multicenter, single-arm, phase I GARNET trial, in which dostarlimab resulted in durable and clinically meaningful responses in EC patients with dMMR/MSI-H [9]. That dostarlimab can maintain this response at longer follow-up is now demonstrated by the results of the third pre-specified interim analysis of the GARNET trial presented at ASCO 2022 [6]. After a median follow-up of 27.6 months, the objective response rate (ORR) in patients with dMMR/MSI-H EC was 45.5 %(Table 1). Median duration of response and median overall survival were not achieved. Most treatment-related adverse events (TRAEs) were grade 1 or 2. The most common grade 3 or higher adverse events were anemia (4.6 %), diarrhea (2.0 %), and increased lipase (2.0 %). The rate of treatment discontinuation due to TRAEs remained low at 8.5 %, and no deaths were recorded as a result of TRAEs. Data on tolerability and adverse effects were consistent with the known safety profile for PD-1-targeted antibodies.
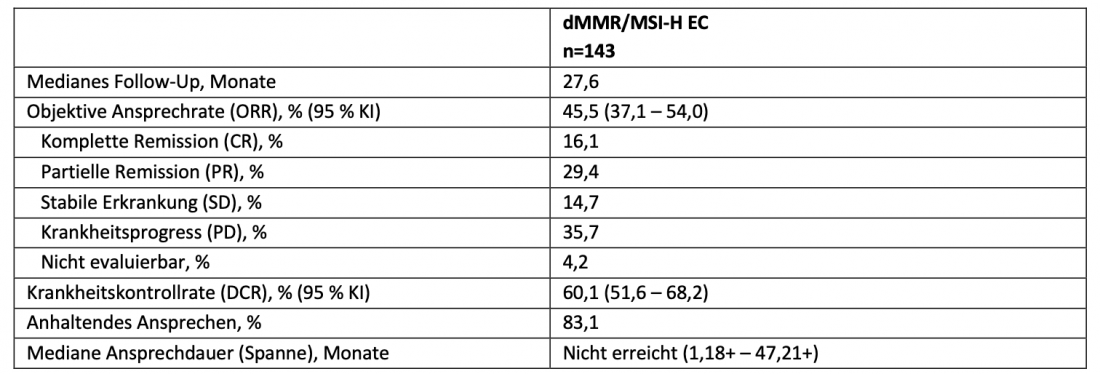
Table 1: Results of the third interim analysis of the GARNET study. Adapted from [6]. dMMR: DNA mismatch repair-deficient; EC: endometrial carcinoma; MSI-H: high microsatellite instability.
Conclusion
Results presented at this year’s ESMO and ASCO annual meetings support the use of PARPi niraparib in the treatment of advanced HRD-positive OC in a broad spectrum of patients [4, 5]. In patients with relapsed or advanced dMMR/MSI-H EC, the third interim analysis of the GARNET trial suggests continued efficacy and a stable safety profile of the anti-PD-1 antibody dostarlimab even after longer follow-up [6].
* with limitatio
Literature
The references can be requested by professionals at GlaxoSmithKline.
Publireportage responsible for content and financed by GlaxoSmithKline AG, Talstr. 3, CH-3053 Münchenbuchsee.
Trademarks are property of their respective owners. ©2022 GSK group of companies or its licensor.
To the brief technical information of Zejula
To the brief technical information of Jemperli
PM-CH-NRP-ADVR-220012-12/2022