There are now four approved JAK inhibitors in Europe: tofacitinib, baricitinib, upadacitinib and filgotinib. While there has not been much news to report on the first two in the past 12 months, there has been a lot of progress, especially with regard to upadacitinib, as was summarized at the Rheumatism Update 2021.
Thus, the first head-to-head study (SELECT-CHOICE) in bDMARD-IR patients was published: 612 patients with active rheumatoid arthritis despite csDMARD therapy and treatment failure to at least one early biologic were randomized in a blinded, placebo-controlled fashion to either abatacept i.v. (n=309) or upadacitinib (n=303) [2]. The primary endpoint was DAS28(CRP) from baseline to week 12. Upadacitinib (UPA) performed better than abatacept (ABA) in all parameters studied. The proportion of patients with DAS28(CRP) remission was nearly 30% with UPA vs. 13% with ABA. Prof. Dr. Andrea Rubbert-Roth from the Department of Rheumatology at the Cantonal Hospital St. Gallen, who is also first author of the study, concluded that UPA therapy is superior to abatacept in terms of efficacy in RA patients with inadequate response to bDMARDs, with a slightly higher incidence of serious adverse events (3.3% vs. 1.6%; 2 VTE and 1 MACE* occurred with UPA; interestingly, herpes zoster occurred equally often in both groups with 4 patients each).
In another study (SELECT-COMPARE), patients whose RA was active despite methotrexate were randomized to UPA 15 mg/day (n=651) vs. adalimumab (ADA, n=327) vs. placebo (n=651). In case of insufficient response (defined as no ACR20 at 14 weeks = nonresponder or CDAI >10 = partial response), they could be switched to the other active agent without pause starting at week 14.
* Major adverse cardiovascular events (= cardiovascular death, non-fatal myocardial infarction, CHD, cerebrovascular events).
Filgotinib “highly potent“ for RA therapy
The result of the switch: the patients who were switched from ADA to UPA did better than those who were switched from UPA to ADA. When switching from adalimumab to upadacitinib, 47% of non-responders and 58% of incomplete responders achieved CDAI-LDA; conversely, these figures were 36% and 45%, respectively. These are the first data to show what can be done when there has been an inadequate response to a JAK inhibitor, Prof. Rubbert-Roth said.
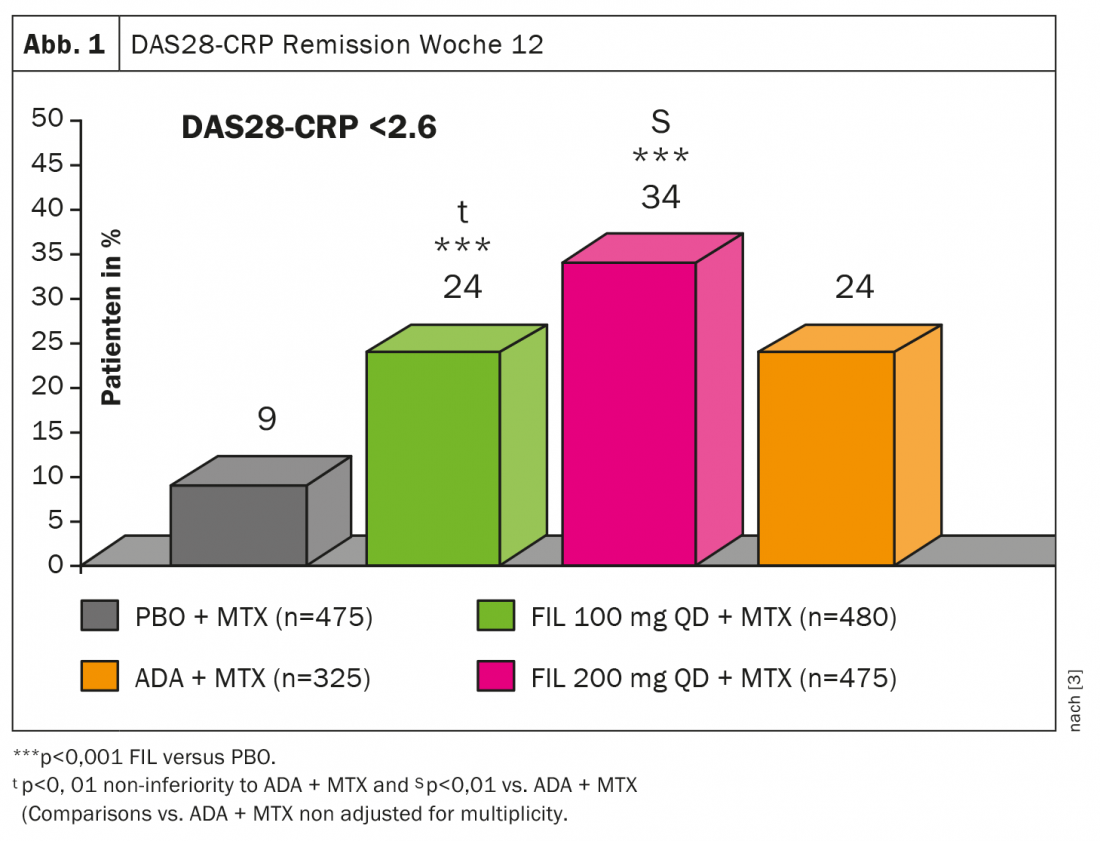
Regarding filgotinib (FIL), data were recently published from the FINCH1 trial, a double-blind, placebo-controlled phase 3 study of 1755 patients with active RA despite methotrexate (MTX) [3]. It was set to FIL 100 mg (n=480), FIL 200 mg (n=475), ADA (n=325), or placebo (n=475), respectively; primary endpoint was ACR20 at week 12. the DAS28(CRP) emission in week 12 was under the combination FIL 200 mg plus MTX better than ADA + MTX or FIL 100 mg + MTX (Fig.1). Even though the superiority was only apparent at the higher dose, “Filgotinib is also a highly potent JAK inhibitor for the treatment of RA,” she concluded.
Study results on biologics
Various new findings have also emerged in research with regard to biologics. One study addressed the question of whether there is a benefit to early initiation with biologics. The NORD-STAR trial, a randomized, multicenter study in 812 patients with early RA published last year, compared four different treatment arms [4]:
- MTX + prednisone (tapering to 5 mg /d) or MTX + SSA + HCQ + intraarticular steroids.
- MTX + certolizumab pegol
- MTX + Abatacept
- MTX + tocilizumab
As a result, there was no significant benefit. MTX + abatacept achieved the highest CDAI remission rate, and conventional combination therapy was noninferior to certolizumab + MTX or tocilizumab + MTX. However, the study was not powered sufficiently to compare the three bDMARD arms. If you did that, you would feel, at least numerically, that the CDAI remission rate under MTX + abatacept is higher than what you see under certolizumab (52.6%) or tocilizumab (48.7%) after all, at 56.3%, Prof. Rubbert-Roth said. However, the study will continue, with the second part examining and comparing 2 de-escalation strategies.
Lymphoma risk not increased
Lymphomas have been a big topic in connection with TNF blocker therapy for years: On the one hand, it has been shown that more lymphomas occur when disease activity is high. On the other hand, increased rates of lymphoma have also been found initially in RA patients on TNF inhibitor therapy, “and I admit I never understood that, because patients on TNF blockers do experience a reduction in disease activity,” Prof. Rubbert-Roth said. Possibly, they thought, it was because they were patients with very long-standing RA. A study from 2020 took another look at this phenomenon by analyzing data from the Swedish RA registry.
In patients with bDMARDs (n=16 392) and those without biologics (n=55 253), all new-onset lymphomas from 2001-2016 were recorded. Analysis of patients treated with a biologic after 2006 now showed, compared with biologic-naive patients, that the former even had a reduced risk of developing lymphoma (adjusted HR 0.69; 95% CI 0.28-0.73). “These are very positive data that certainly help support biologics in benefit,” the rheumatologist said.
Finally, Prof. Rubbert-Roth also reported positive results on the use and safety of biologics in RA patients with a history of malignancy. A meta-analysis based on 12 trials involving 13,598 RA patients, 10 trials of TNF blockers, and 3 trials of rituximab examined the relative risk of recurrence or second cancer [5]. Here, the results showed that the risk was comparable among TNF blockers, rituximab, and csDMARDs and was independent of the duration and interval of TNF inhibitor therapy.
Take-Home Messages
- Upadacitinib: interesting switch data from SELECT-COMPARE trial, first head-to-head study in bDMARD-IR patients vs. abatacept
- Filgotinib: superiority vs. ADA only at the higher dose.
- There was no superiority in bDMARD-containing combination therapies for the treatment of early RA compared with the csDMARD strategy.
- The risk of lymphoma in RA patients remains elevated compared with the normal population, but neither TNF inhibitors nor non-TNF biologics increase the risk. The trend even shows a reduced risk of lymphoma under biologic therapy.
- In case of a history of malignancy, TNF blockers, rituximab, and csDMARDs are comparable in terms of risks for recurrence or second tumor.
Literature:
- RA: Therapy, Rheumatism Update 2021 (online), Mar. 12, 2021.
- Rubbert-Roth A, et al: Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med 2020; 383: 1511-1521; doi: 10.1056/NEJMoa2008250.
- Combe B et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis 2021; doi: 10.1136/annrheumdis-2020-219214.
- Hetland ML, et al: Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial. BMJ 2020; 371: m4328; doi: 10.1136/bmj.m4328.
- Xie W, et al: A meta-analysis of biologic therapies on risk of new or recurrent cancer in patients with rheumatoid arthritis and a prior malignancy. Rheumatology 2020; 59: 930-939; doi: 10.1093/rheumatology/kez475.
InFo PAIN & GERIATRY 2021; 3(1): 34-36 (published 7/3/21, ahead of print).