The standard treatment for tuberculosis remains 2 months of quadruple therapy with rifampicin, isoniazid, pyrazinamide, and ethambutol, followed by 4 months of therapy with rifampicin and and isoniazid. The average success rate of this regime is about 85%. However, this treatment, which has been established for decades, is in a state of flux and has already given way to new recommendations in some cases.
In the 1990s, four active ingredients appeared on the market: moxifloxacin, vancomycin, linezolid and clofazimine. Their indications were originally for diseases such as anaerobic infections (moxifloxacin) or leprosy (clofazimine), but they were later also recognized as effective in the treatment of tuberculosis (TB). Currently, these agents are all part of the WHO recommendations for the treatment of multidrug-resistant tuberculosis (MDR-TB). In the past decade, new active ingredients have also been developed with bedaquiline and delamanid (EU approval 2014), and finally in the recent past pretomanid (EU approval 2020, only in combination with bedaquiline and linezolid).
There are several targets that can be targeted in Mycobacterium tubeculosis. The new concept is to downregulate the energy of M. tuberculosis by inhibiting electron transport. ATP synthase (bedaquiline), NADH dehydrogenase (clofazimine), plus two compounds under development that inhibit cytochromes C and D – if these four targets are all inhibited, there is theoretically no more electron transport, which could dramatically reduce the duration of therapy in TB, explained Prof. Dr. Dr. h.c.. Christoph Lange, Research Center Borstel, Leibniz Lung Center, Borstel (D).
Non-inferiority with shorter duration of therapy.
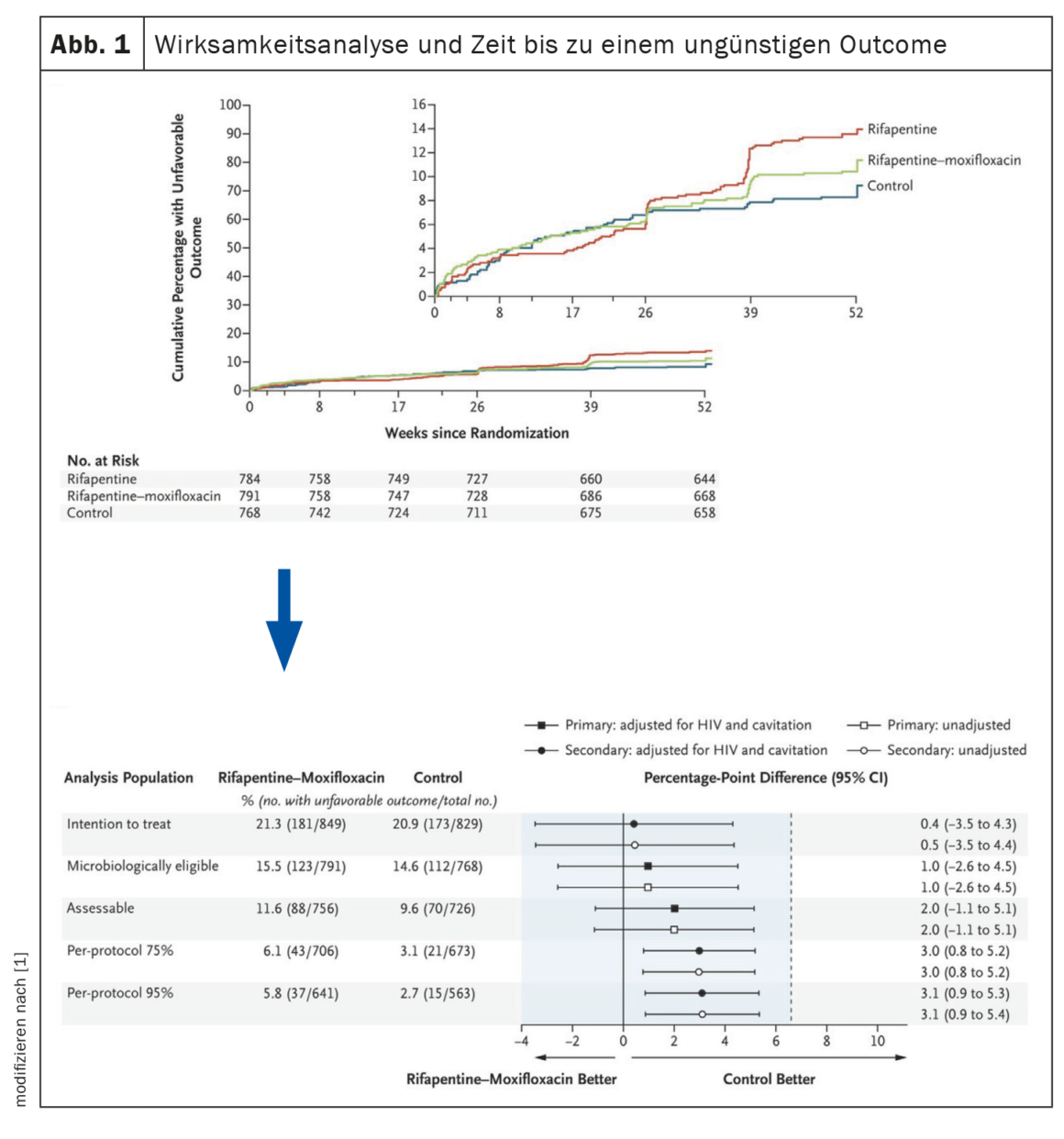
In a sentinel study published in 2021 [1], standard treatment (6 months of rifampicin + isoniazid plus an initial 4 months of pyrazinamide + ethambutol) was compared with two regimens in which some agents were exchanged and the duration of therapy was shortened: The first arm consisted of 4 months of isoniazid, 2 months each of pyrazinamide and ethambutol, and 4 months of rifapentine. The regimen in the second arm consisted of 4 months of isoniazid, 2 months of pyrazinamide, and 4 months each of rifapentine and moxifloxacin (Fig. 1). The latter comparator arm proved noninferior to the 6-month regimen. Thus, swapping ethambutol for moxifloxacin and rifampicin for rifapentine allows the duration of TB therapy to be reduced to 4 months, with the same outcome. In the U.S., this regimen is now recommended by the Centers for Disease Control and Prevention (CDC) for the treatment of pulmonary tuberculosis. The problem is that although rifapentine was developed in the 1960s and is approved in the United States, it is available in only 6 of 43 European countries (12%) [2].
Another study [3] investigated a shorter duration of treatment in African and Indian children with paucibacillary tuberculosis. “Paucibacillar means the bacteria grow in culture, but the acid-fast rods are not seen in smear microscopy, which is more or less the standard in children under 5 because they don’t produce enough sputum,” Prof. Lange explained.
This study also compared the standard regimen with a shortened 4-month regimen, with only isoniazid and rifampicin administration reduced from 6 to 4 months, in some cases even with ethambutol omitted (for reasons of lack of availability). Again, the results were non-inferior to the standard, which has already led WHO to recommend this new 4-month regimen for the treatment of paucibacillary TB in children.
“Dramatic improvement”
Between 2009 and 2019, WHO has seen a 20% increase in registered MDR-TB cases globally each year (after which there were diagnostic delays due to the COVID-19 pandemic). Already two years ago a publication [4] could show a “dramatic change in MDR-TB”, as Prof. Lange explained: The new agent pretomanide (200 mg/d) in combination with linezolid in high dose (1200 mg/d – 600 mg in the morning + 600 mg in the evening) and bedaquiline (400 mg/d for 2 weeks, then >200 mg/3× weekly) over a period of only 6 months in patients with the highest concentration of drug-resistant bacteria receiving this regimen resulted in treatment success in 90%. However, serious adverse events due to the high dose of linezolid also occurred in 89% of cases, which terminated treatment. However, based on the success of the therapy, further research groups investigated how the combination of these three agents could best be administered.
In the ZeNix study, patients received the pretomanid/linezolid/bedaquiline regimen for 6 months as in the previous study, with the only difference being that linezolid was administered at a lower dose [5]. Participants with MDR- or XDR-TB were divided into 4 groups and received
- 1200 mg L (linezolid) over 2 and 6 months, respectively.
- 600 mg L over 2 or 6 months
All but the last group achieved a success rate of at or above 90%. Only the group receiving 600 mg of linezolid for 2 months achieved only 84%.
These findings prompted WHO to issue new recommendations for the treatment of MDR-TB in May 2022: the new regimen consisting of pretomanid, bedaquiline, and linezolid is to be given for only 6 months in the future, with linezolid dosed at 600 mg per day. However, if patients have previously received one of these agents for more than one month, it should no longer be administered. The alternative is a 9-month regimen, which is recommended especially for patients living in regions where pretomanid is not available (which is currently still the case in much of Europe). Only if there are reasons to exclude patients from these two options is the 18-month previous standard regimen recommended.
Source: Symposium: Respiratory infections; Lecture: New drugs and regimens in tuberculosis. European Respiratory Society Congress, Barcelona, Sept. 4, 2022.
Literature:
- Dorman SE, Nahid P, Kurbatova EV, et al: Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med 2021; 384: 1705-1718; doi: 10.1056/NEJMoa2033400.
- Guglielmetti L, Günther G, Leu C, et al: Rifapentine access in Europe: growing concerns over key tuberculosis treatment component. Eur Respir J 2022; 59: 2200388; doi: 10.1183/13993003.00388-2022.
- Turkova A, Wills GH, Wobudeya E, et al: Shorter Treatment for Nonsevere Tuberculosis in African and Indian Children. N Engl J Med 2022; 386: 911-922; doi: 10.1056/NEJMoa2104535.
- Conradie F, Diacon AH, Ngubane N, et al: Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med 2020; 382: 893-902;
doi: 10.1056/NEJMoa1901814. - Conradie F, Bagdasaryan TR, Borisov S, et al: Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med 2022; 387: 810-823; doi: 10.1056/NEJMoa2119430.
InFo PNEUMOLOGY & ALLERGOLOGY 2023; 5(1): 28-29 (published 2/22/2013, ahead of print).