The autoimmune disease TTP is rare, life-threatening and difficult to treat. The results of a large phase III trial involving Inselspital Bern are now drawing attention to a new therapeutic option: caplacizumab.
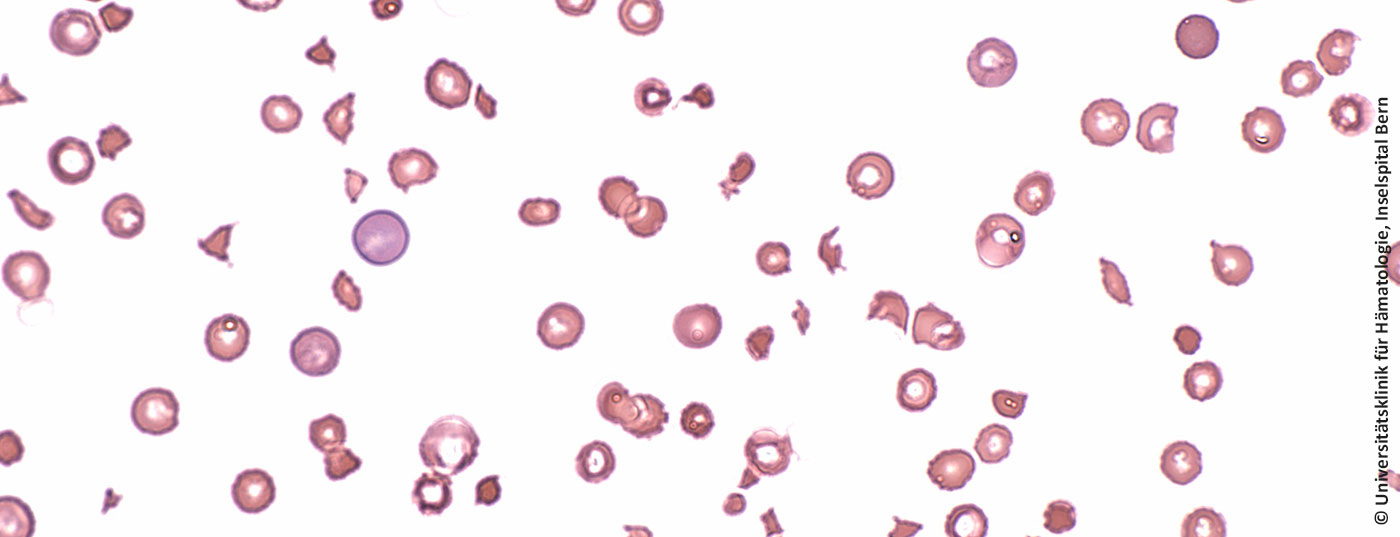
With an annual incidence of two to three new cases per million, thrombotic thrombocytopenic purpura (TTP) occurs rarely. If it is not treated, it leads to death within a few days. Mostly younger, previously healthy people, predominantly women, are affected.
In healthy humans, the protease ADAMTS13 ensures cleavage of the protein “Von Willebrand factor” (VWF) and thus prevents platelets from sticking to the VWF. ADAMTS13 is missing in the autoimmune disease TTP. Clots form and organs become less perfused, which can lead to kidney failure, heart attack or stroke.
To date, daily plasmapheresis (extraction of autoantibodies, addition of ADAMTS13) in combination with immunosuppressants was considered the gold standard in the treatment of TTP. Nevertheless, 10-20% of patients die during an acute episode; more than half suffer permanent organ damage and disability, often of a neurological nature. Relapses are frequent.
An international phase III trial with participation of the Department of Hematology of the Inselspital Bern has now confirmed the efficacy of a promising therapeutic approach. The nanobody caplacizumab, which targets VWF, ensures that VWF and platelets do not stick together, thus reversing the disease in a matter of days and preventing further organ damage. The study enrolled 145 patients, half of whom received the Nanobody or placebo for 30 days in addition to plasma exchange. For 75% of the verum group, the acute phase of TTP ended after only 2.95 days (placebo group: 4.5 days). In addition, nanobody subjects required less plasmapheresis (median 5 vs. 7) and could be discharged earlier. The adverse event profile was similar in both groups, although minor bleeding was more common with Nanobody treatment, as expected (65% vs. 48%).
Source: Scully M, et al: Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med 2019; doi: 10.1056/NEJMoa1806311 [Epub ahead of print].
HAUSARZT PRAXIS 2019; 14(1): 2