The new transfemoral TAVI systems, either in development or already certified, address potential vulnerabilities and limitations, such as paravalvular insufficiency or risk of vascular complication. This article, based on a presentation at the 4th Bern Heart Valve Symposium, provides an insight into the latest developments.
Percutaneous transcatheter aortic valve implantation (TAVI) has become a recognized and proven method in the treatment of severe valvular aortic valve stenosis since the first implantation in 2002. The minimally invasive concept has been continuously improved in recent years. Different access routes as well as various balloon or self-expandable flap systems were tested or further developed. Based on clinical data from randomized trials as well as registries, TAVI is superior to medical therapy and on par with conventional cardiac surgery in high-risk patients. The transfemoral approach offers the advantage of a procedure under local anesthesia without general anesthesia and is used in the majority of cases with suitable anatomy. Based on the clinical results, TAVI therapy was included in the European Society of Cardiology guidelines in 2012 with a Class I B recommendation in inoperable and a Class IIa B recommendation in high-risk patients with severe aortic valve stenosis. Clinical experience with TAVI procedures is based on the use of the Edwards Sapien as well as the Medtronic Corevalve bioprosthesis, which have established themselves as primary devices in clinical practice for transfemoral procedures. The total number of procedures has increased rapidly worldwide, but also in Switzerland. Since the first TAVI in 2007 in Bern, approximately 650 procedures per year have been performed in Switzerland.
New CE certified systems
Edwards Sapien 3: The Edwards Sapien 3 (Edwards Lifesciences Inc., CA, USA) represents the further development of the Edwards Sapien XT prosthesis and, like its predecessor, has a cobalt-chromium alloy stent frame and a bovine pericardium heart valve. While the Sapien 3, unchanged from the previous models, is fixed with a balloon in the native aortic valve annulus, it brings as a special new development for the first time a sealing seam made of polyethylene terephthalate (PET) in the lower outer area of the heart valve (Fig. 1a).
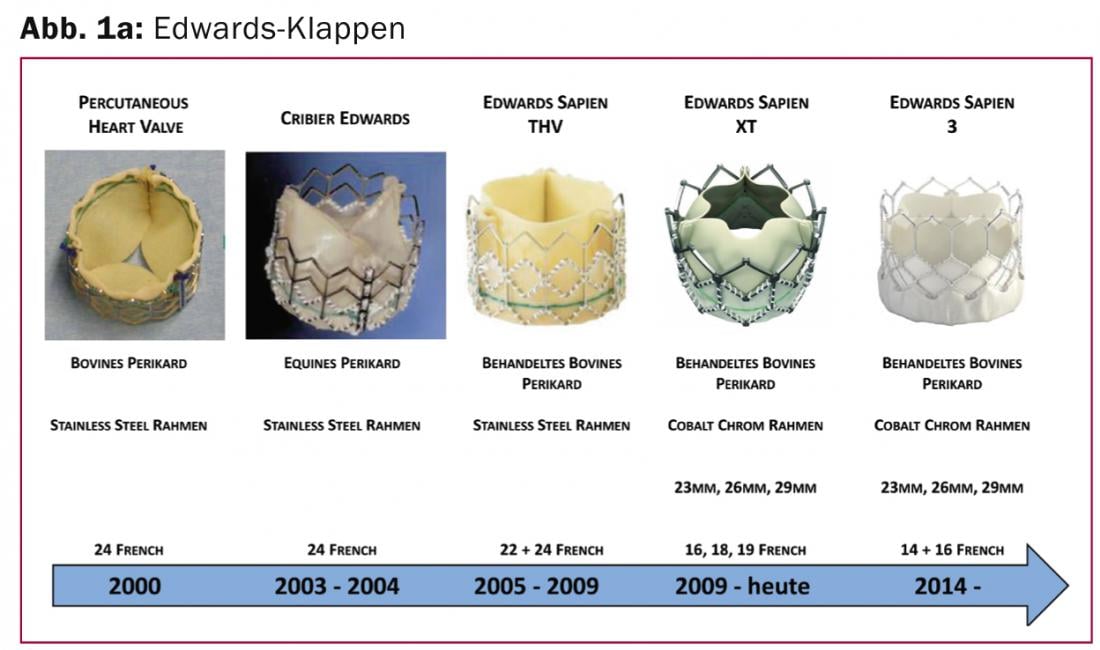
This sealing rim is designed to fill gaps between the prosthesis and the degenerative native valve, preventing paravalvular leakage. A change in the design of the valve stent also allows implantation through smaller airlock sizes and easier access to the coronary arteries. In addition to the further development of the valve prosthesis, the femoral implantation catheter was also fundamentally revised and reduced in diameter. Currently, only a 14-French (inner diameter) sheath is required for implantation of a 23- and 26-mm valve, and a 16-French sheath for the 29-mm valve. The system has recently received CE approval and can be used commercially.
Boston Scientific Lotus flap: The self-expandable Lotus flap system (Boston Scientific, Natick, MA, USA) consists of a bovine pericardial flap inserted into an elongated nitinol wire mesh. During implantation of this prosthetic valve, the stent shortens and thereby anchors to the native aortic valve annulus. In addition to the lack of hemodynamic compromise during implantation, the controlled and slow release has the advantage, among others, that the system can be positioned very precisely and, if necessary, completely reloaded in the body even after full deployment. As another special feature, the Lotus valve has adaptive sealing material in the lower part of the prosthesis, which causes a reduction in paravalvular aortic valve regurgitation. The Lotus valve is implanted via an 18- or 20-French catheter, is CE certified, and has been commercially available in 23 mm and 27 mm sizes since October 2013.
St. Jude Medical Portico: The Portico™ heart valve (St Jude Medical, St Paul, MN, USA) is a self-expandable TAVI prosthesis consisting of a nitinol stent frame into which a bovine pericardial valve with a porcine pericardial border is sewn. Visually, the valve resembles the Medtronic Corevalve bioprosthesis, although the shape differs primarily in the distal, annular portion. In addition, the cells of the stent frame are larger and the radial force is lower. The concept is based on the fact that this should reduce the risk of electrical conduction disturbance and improve the adaptation of the prosthesis to the native anatomy. The valve plane also comes to lie intraannularly. The recently approved Portico™ (23 – 25 mm prosthesis sizes) is inserted via an 18-French catheter and can be partially repositioned.
Direct Flow Medical: The recently CE- certified Direct Flow Medical (Santa Rosa, CA, USA) prosthetic heart valve features a completely different design than other TAVI bioprostheses. It has no metallic components, has an aortic and ventricular ring which prevents paravalvular insufficiencies, and can be checked for position and tightness after complete release and repositioned if necessary. Clinical experience with this bovine pericardial valve is still limited; annulus dimensions up to 28 mm can be treated with it.
New, non-CE-certified systems used in clinical trials
Edwards Centera: The Edwards Centera (Edwards Lifesciences Inc., CA, USA) is a self-expandable prosthetic valve consisting of a nitinol stent frame with a bovine pericardial valve. The special shape of the valve stent aims to facilitate self-alignment and centering of the valve prosthesis in the native valve annulus and uses a polyethylene terephthalate (PET) sheath in the lower part to achieve sealing and prevention of paravalvular leakage. The very controlled release is controlled by an electric motorized mechanism. Despite high radial force, repositioning and removal of the valve prosthesis is also possible during implantation in case of suboptimal positioning. The Edwards Centera is being tested in clinical trials in 23 mm, 26 mm and 29 mm sizes and can be inserted via a 14-French guiding catheter. The first results of this valve prosthesis were positively received.
Medtronic CoreValve Evolut R: The Medtronic CoreValve Revalving System (Medtronic, Minneapolis, MN, USA) (Fig. 1b) was the second system successfully introduced as a transcatheter system for the treatment of severe aortic stenosis.
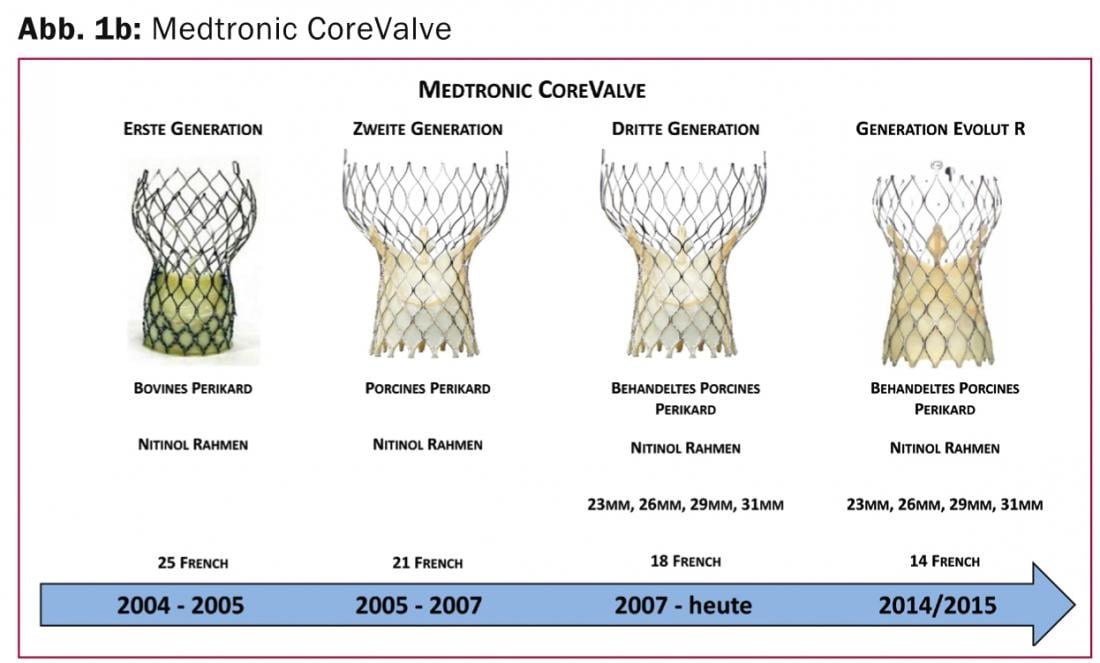
Since its CE Mark certification in 2007, it has been regularly used worldwide and features a supra-annular valve position and self-expandable implantation technology. The next generation (Evolut R) aims at simplified and repeated positioning of the prosthesis, i.e. the system can be retracted into the catheter again and repositioned in case of suboptimal position. This new technology has already been used clinically in studies and consists of a slightly modified nickel-titanium alloy (Nitinol) with improved cell geometry as well as a new structure of the outflow area and a reduction of the prosthesis size by 10%. The modified design should allow improved fit and alignment of the valve prosthesis even in specially angulated aortic root anatomy and covers the widest range of annulus sizes (18 -29 mm). Approval for this improved system, which uses porcine pericardium, is expected in 2014 / 2015.
Further new-generation transfemoral valve systems: In the near future, additional TAVI systems will expand the range (Fig. 2) .
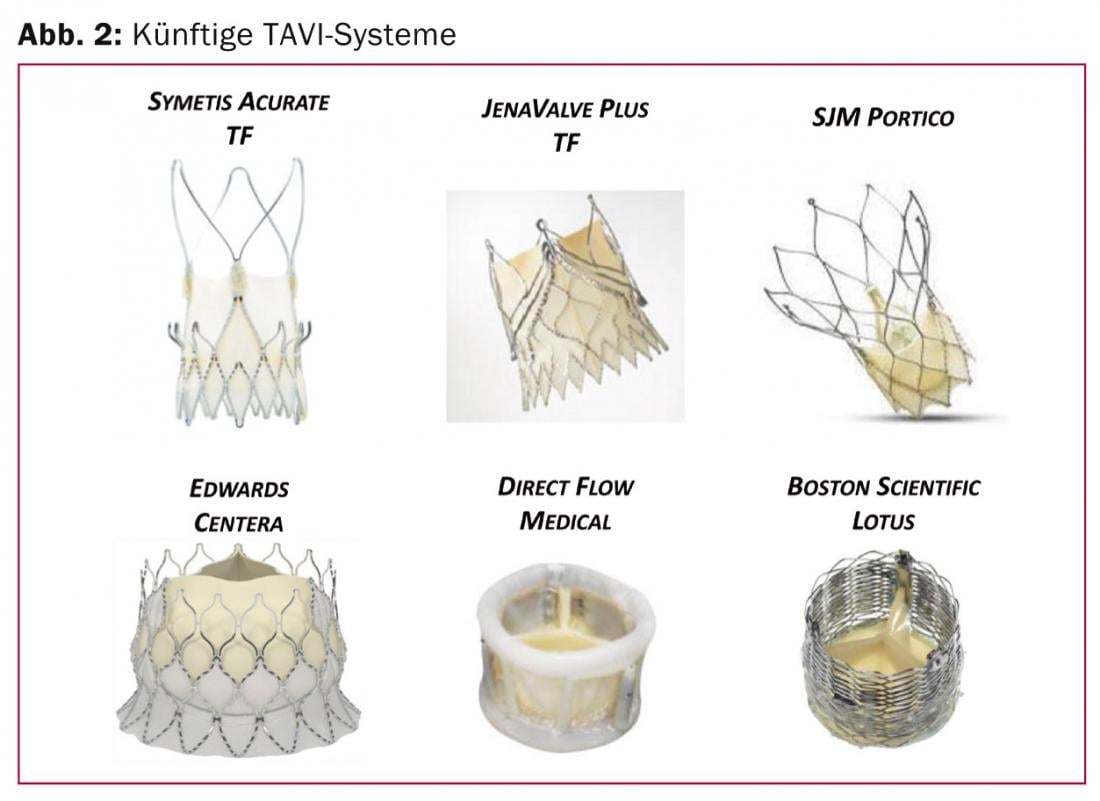
Predominantly, self-expandable systems are being developed which, depending on the anatomy, can also be inserted directly into the native annulus without pre-dilation. These are systems such as the Symetis Acurate TF (Symetis Inc, Ecublens, Switzerland) or the JenaValve Plus (Munich, Germany), which have already been approved via the transapical approach and are now being clinically tested for the transfemoral approach. They feature an alternative anchoring technique and innovative self-centering and stabilizing mechanisms. Anchoring mechanisms using native valve leaflets and valve pockets avoid contact with the subvalvular area and thus also minimize the risk of electrical conduction disturbances and the need for definitive pacemaker implantation. The latter should be mentioned as a disadvantage for self-expandable systems that are partially located in the left ventricular outflow tract.
The Swiss-TAVI Registry
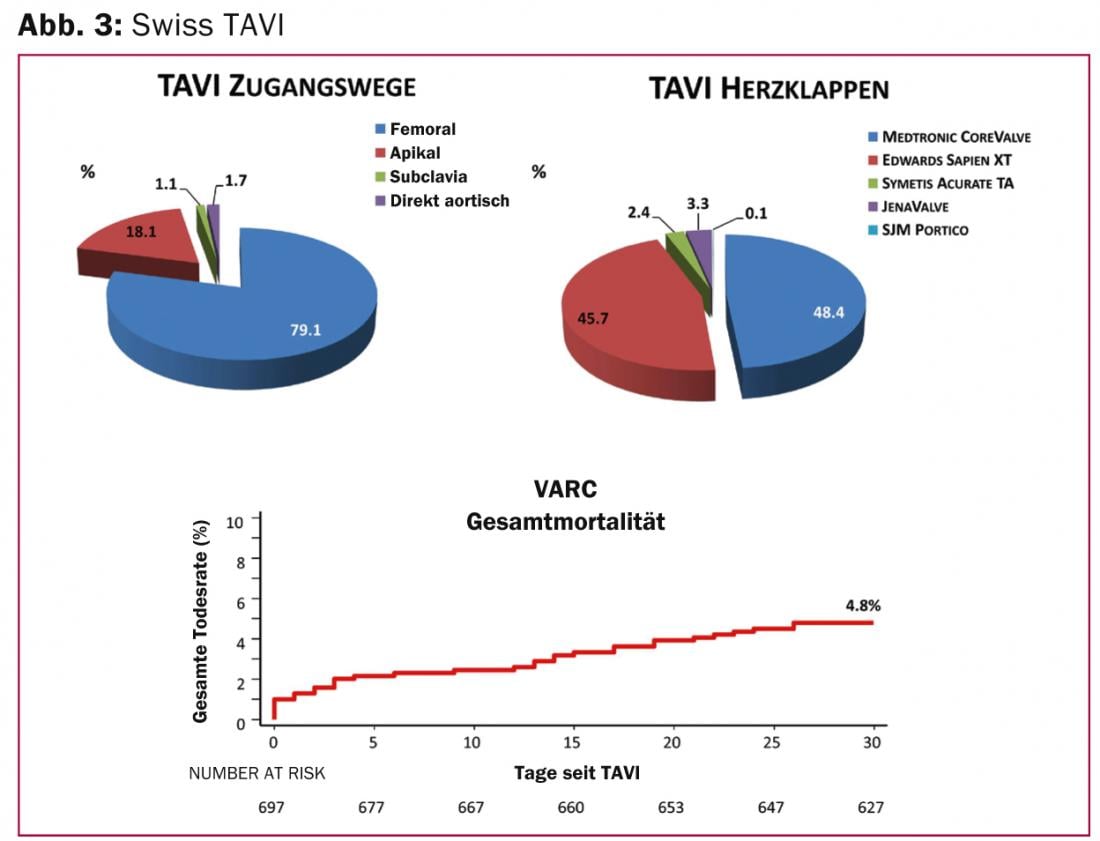
The Swiss TAVI Registry is a national interdisciplinary project of the Swiss Working Group for Interventional Cardiology and the Swiss Society for Cardiac Surgery, and was initiated in 2011 to define a corresponding national quality standard of TAVI therapy and to be able to offer this uniformly in Switzerland. Currently, approximately 50-60 TAVI interventions are performed per month in Switzerland. Patients are high-risk (calculated 30-day mortality risk according to STS score of 8.2%), severely symptomatic (73% NYHA III and IV), and 82 years old on average. In most cases a femoral implantation route is used (79%), less frequently the transapical, the direct aortic or the access via the subclavian artery (Fig. 3) . The mortality rate of 4.8% after 30 days was below the expected rate. Cerebrovascular events occurred in 3.3% (2.5% with permanent deficits) and myocardial infarction in 0.4% within the first 30 days after intervention. The most common complications were vascular (11.8%), and local bleeding (16.6%) and pacemaker implantation (20.5%).
Summary
Minimally invasive catheter treatment of severe aortic valve stenosis has emerged as an alternative to surgical aortic valve replacement and is now an established therapy for selected patients. Continued technical innovations and advancements address or minimize previous limitations such as paravalvular aortic regurgitation and access complications. As a result, the new generation of TAVI valve prostheses and implantation systems promises to further improve clinical endpoints by markedly reducing peri-procedural complications. Ongoing clinical studies will show whether these high expectations can be met. This will also determine whether the indications for the use of TAVI bioprostheses can be expanded in the further future.
Prof. Dr. med. Peter Wenaweser
Stefan Stortecky, MD
Source: “New Transfemoral TAVI Systems and Swiss TAVI Registry”, presentation at the 4th Bern Heart Valve Symposium, February 13, 2014, Bern.
Literature:
- Wenaweser P, et al: Short-term clinical outcomes among patients undergoing transcatheter aortic valve implantation in Switzerland: the Swiss TAVI registry. EuroIntervention 2014 (March [epub ehead of print]).
- Blumenstein J, et al: Recent Advances in Transcatheter Aortic Valve Implantation: Novel Devices and Potential Shortcomings. Current Cardiology Reviews 2013; 9: 274-280.
- Leon MB, et al: Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17): 1597-1607.
- Smith CR, et al: Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364(23): 2187-2198.
- Wenaweser P, et al: Clinical outcomes of patients with severe aortic stenosis at increased surgical risk according to treatment modality. J Am Coll Cardiol 2011; 58(21): 2151-2162.
CARDIOVASC 2014; 13(2): 39-42