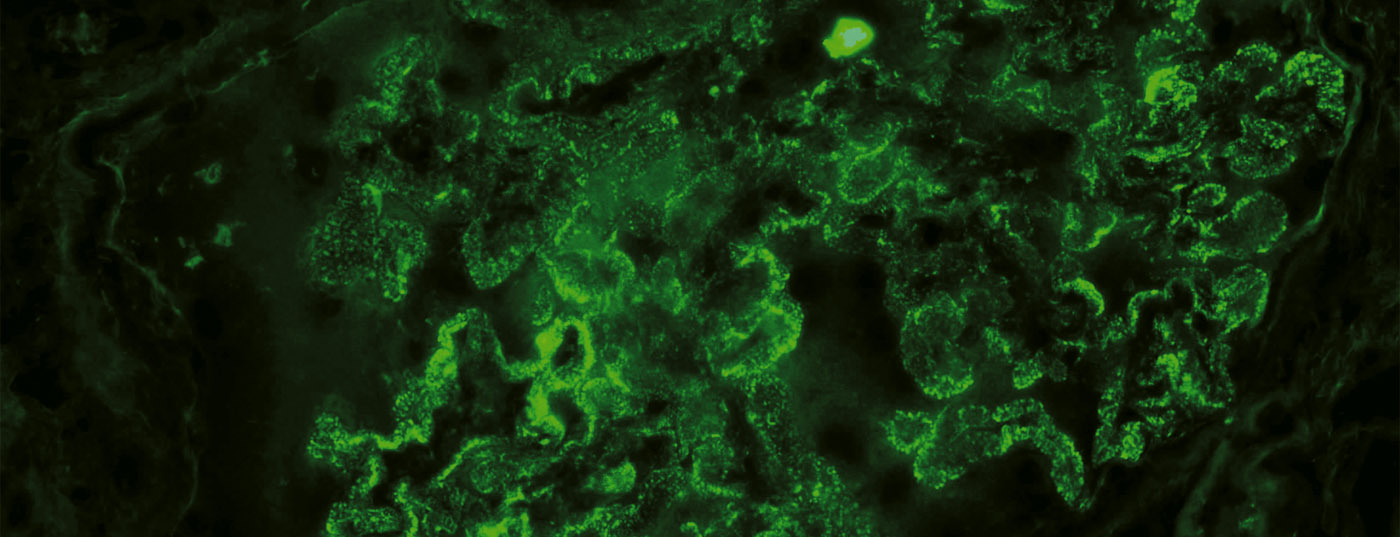
Glomerular diseases are important causes of end-stage renal failure. Pathogenetically, immune-mediated processes are in the foreground. Thanks to advancing knowledge of the pathophysiology and modern genetic engineering methods, there is hope for a therapy that is as targeted as possible and has few side effects. New treatment approaches for glomerular diseases are briefly reviewed in this article and some substances are presented as examples.
Glomerulopathies (GP) are an important cause of chronic and end-stage renal failure. In the U.S., diabetic nephropathy ranks first and non-diabetic GP ranks third in etiology for terminal kidney failure (TNV). The pathogenesis of this heterogeneous group of diseases is mostly immune-mediated. General principles of therapy include specific therapy in addition to supportive therapy in certain cases. This generally consists of relatively untargeted immunosuppressants, some with cumulative toxicity, which proves problematic in GP, which is often recurrent. Thanks to the increasing elucidation of pathogenetic processes of different GP in the last decades as well as modern genetic engineering methods, there is hope for a targeted therapy oriented to the pathomechanism.
This overview is intended to name recent developments in the therapy of glomerular diseases without claiming completeness. Practice-relevant topics as well as innovative therapeutic approaches and concepts will be highlighted.
Supportive therapy
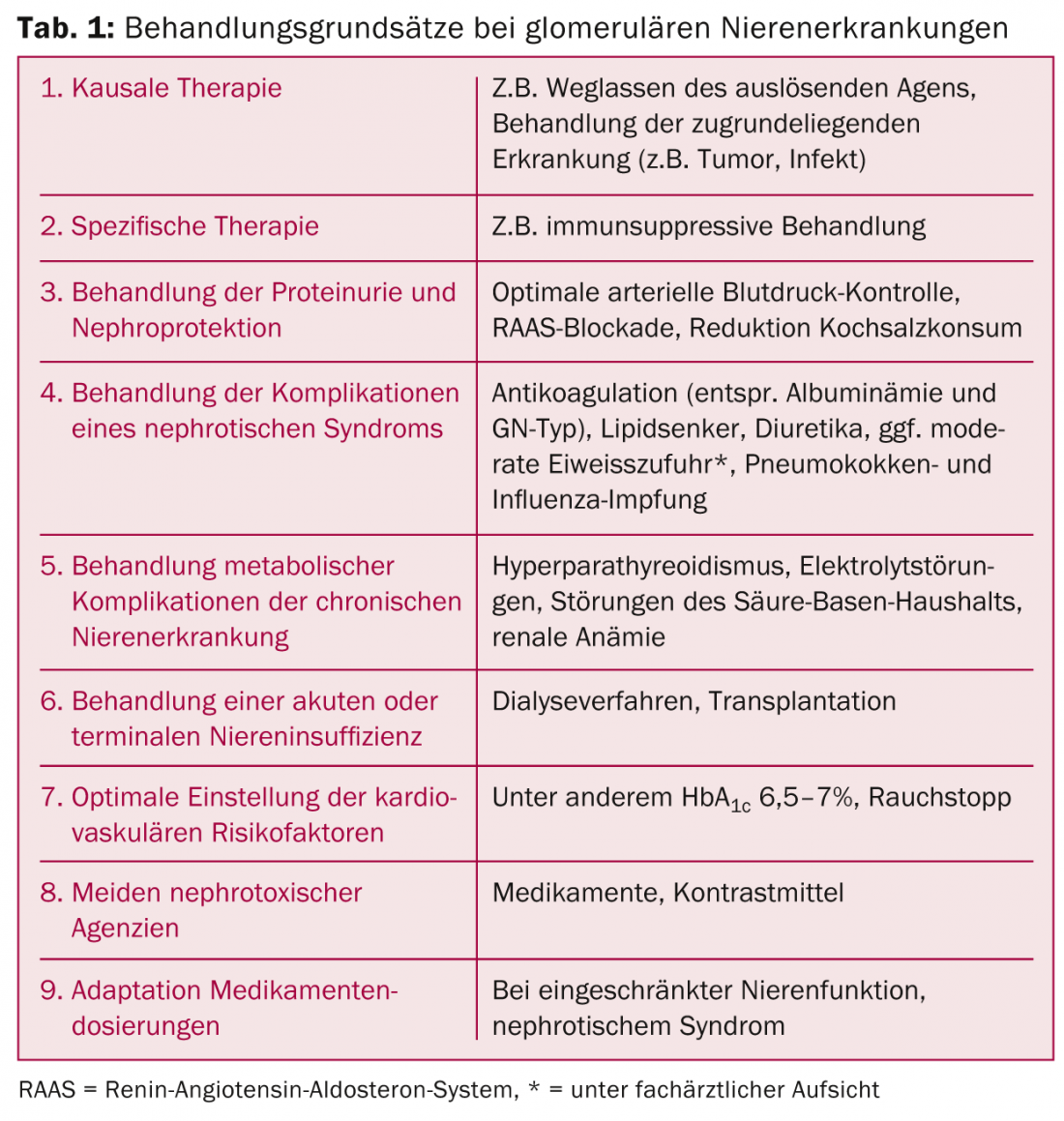
As with all chronic kidney diseases, optimally implemented supportive therapy is essential. In some cases, immunosuppressive treatments can be avoided and renal and/or overall prognosis can be improved. This is where the primary care provider has a key role to play. Therapeutic cornerstones can be found in Table 1.
In the treatment of proteinuria, in addition to strict control of arterial blood pressure values (<130/90 mmHg for nondiabetics, <130/ 85 mmHg for diabetics) [1] and limited saline consumption (5-6 g/d), drug inhibition of the renin-angiotensin-aldosterone system (RAAS) is indicated. ACE inhibitors and sartans are maxed out after tolerance (higher maximum dose than for hypertension treatment). Evidence in recent years has questioned the value of dual RAAS blockade [2,3]. Indeed, diabetics in particular show higher risks of hyperkalemia and acute renal failure with concomitant nonimproved cardiovascular prognosis despite additive effects on proteinuria. The new introduction of such medication in renal patients can therefore no longer be generally recommended at present. The combination of an ACE inhibitor or sartan with aldosterone antagonists has a synergistic effect on proteinuria with, however, an increased risk of severe hyperkalemia episodes, especially in the setting of impaired renal function.
B-cell directed therapy
Because of their central role in the pathogenesis of autoimmune diseases (production of pathogenic autoantibodies, antigen presentation, production of proinflammatory cytokines), B lymphocytes have been explored as a therapeutic target in various GP in recent years.
Rituximab
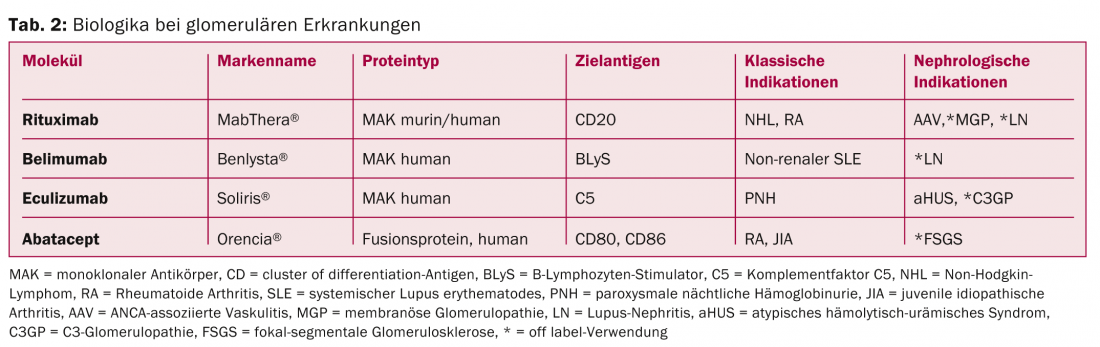
Rituximab (RTX) is a chimeric (mouse/human) monoclonal antibody directed against the surface antigen CD20 of B lymphocytes (Table 2) . Binding to this antigen induces cell lysis and leads to depletion of B cells in peripheral blood. Since its introduction for the treatment of non-Hodgkin’s lymphoma in 1997, the use of RTX has expanded to autoimmune diseases such as rheumatoid arthritis and transplant medicine. RTX has been previously applied in the treatment of various GP [4]. Currently, the most important indications studied are ANCA-associated vasculitides, lupus nephritis, and idiopathic membranous glomerulopathy.
ANCA-associated vasculitides (AAV): Therapy of AAV with RTX was approved in Switzerland in 2012. Approval was based on two randomized-controlled trials (RCTs) including patients with renal involvement of AAV; the trials showed comparable efficacy (possibly even higher efficacy in recurrences) with (unexpectedly) comparable rates of side effects to standard therapy with cyclophosphamide and high-dose steroids [5,6]. According to international guidelines, this therapy can be considered as an alternative to standard therapy. Due to a lack of long-term data, the substance is currently used in practice especially in contraindications to conventional therapy (e.g., desire to have children), in relapses or refractory cases.
Lupus nephritis (LN): RTX has been used successfully in numerous observational studies and case series (especially in severe relapses, relapses, in refractory situations, and in contraindications to conventional therapy) to treat LN. Two RCTs of RTX as add-on therapy in patients with active renal or extrarenal systemic lupus erythematosus show comparable remission rates to placebo therapy [7,8]. Despite these disappointing results, which experts attribute to the study design and other factors, and encouraged by follow-up data and practical experience, many clinicians continue to treat off-label with RTX, especially in the situations mentioned. Further studies evaluating RTX for the treatment of LN based on different immunosuppressive regimens are planned.
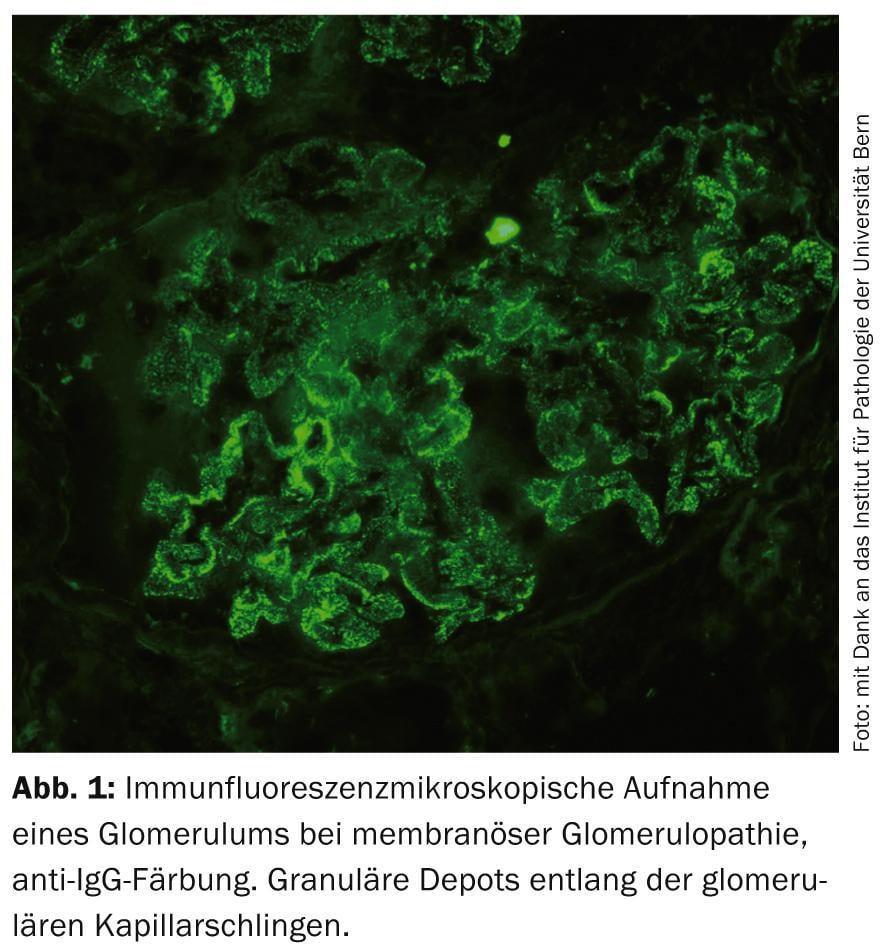
Idiopathic membranous glomerulopathy (MGP): MGP is one of the most common primary GP (Fig. 1) . Groundbreaking research findings in the last decade have provided evidence that MGP is an autoimmune disease with formation of autoantibodies against podocytic antigens [9]. Against this background, therapy with RTX is pathophysiologically interesting. RTX has been successfully used to date in numerous observational studies with a wide variety of administration regimens to treat mainly refractory cases [10]. Controlled and long-term data are lacking at this time, but several RCTs comparing this agent to supportive or other immunosuppressive therapies are ongoing. In practice, RTX is currently used off-label in MGP primarily for detected autoantibodies.
The widespread use of RTX shows generally good tolerability. The most common side effects include infusion reactions (therefore administration of premedication is necessary), neutropenia, hypogammaglobulinemia, and infections; isolated cases of progressive multifocal leukoencephalopathy have been described.
Plasma cells are not depleted by RTX, and tissue-derived B cells may be only partially depleted. Limited efficacy remains in nephrotic syndrome, polymorphisms of the Fc-γ receptor, and high levels of B-cell stimulating factor (BLyS). In addition, a small proportion of B cells, regulatory B cells with anti-inflammatory activity, are also depleting.
Belimumab
Belimumab (BEL) is a humanized monoclonal antibody that binds to soluble BLyS, inhibiting B cell survival and differentiation into plasma cells. BEL was recently approved for the treatment of extrarenal SLE. Posthoc analyses of the data show a possible decreased rate of renal recurrence in BEL-treated patients [11]. Studies in renal SLE with or without administration of RTX (as synergistic B-cell immunomodulation) will help define the role of BEL for nephrologists.
Eculizumab
Eculizumab (ECU) is a humanized monoclonal antibody against complement factor C5, which is at the common end of the complement activation cascade. Initially used to treat paroxysmal nocturnal hemoglobinuria, the drug has been approved for the treatment of atypical hemolytic uremic syndrome since 2011. Another disease entity based on dysregulation of the alternative complement pathway are the C3 glomerulopathies (C3GP), which have recently been distinguished from the classical immune complex-mediated (e.g., in infections, neoplasms) membranoproliferative glomerulonephritides thanks to recent findings [12]. In this context, the successful use of ECU was documented in case reports. Because of the frequently complement-mediated damage, ECU has also been tested in other GN such as IgA nephropathy, LN, and MGP. Hopes for widespread use are currently dampened by the extremely high costs of such a potentially lifelong therapy.
Abatacept
The fusion protein Abatacept (ABT) binds to the antigen CD80 on T cells, inhibiting T cell costimulation by antigen-presenting cells. To date, data exist primarily in LN (RCT, negative) and AAV (observational, positive). Interestingly, induction of CD80 surface molecules in podocytes has recently been demonstrated in the case of proteinuric renal disease. ABT has thus been successfully used in five patients with primary focal segmental glomerulosclerosis (FSGS) and recurrent FSGS after renal transplantation [13]. These promising data await confirmation in further studies.
Topical steroids in IgA nephropathy.
IgA nephropathy (IgA-NP) is the most common GP worldwide. Mesangial deposits of insufficiently glycosylated IgA antibodies, together with induced autoantibodies, lead to glomerular inflammation. The cause is thought to be a dysregulation of the mucosal immune system leading to a defective immune tolerance of e.g. alimentary or bacterial antigens. In a Swedish proof of principle study, budesonide in a new formulation with release in the ileocecal region was used to treat patients with IgA-NP and a reduction in proteinuria was observed. Interesting besides the novel therapeutic concept is the minimization of steroid-induced systemic effects [14]. A phase II trial is currently ongoing.
Adrenocorticotropic hormone
Adrenocorticotropic hormone (ACTH) was used to treat nephrotic syndrome as early as the 1950s, but then abandoned in favor of oral glucocorticoids, which are easier to administer. Thanks to an accidental rediscovery in a lipid study in nephrotic MGP patients, it has been increasingly used in proteinuric GP in recent years (synthetic ACTH in Europe, animal-derived ACTH in gel form in the US). The mechanism of action appears to go beyond induction of cortisol production and to be mediated via binding to melanocortin receptors on podocytes. Observational data exist to date in minimal change disease and primary FSGS, with an additional pilot RCT in MGP with promising results [15]. Steroid-like side effects have been described. Here, too, the high cost of therapy is a limiting factor.
Eprodisate
AA amyloidosis is a multisystem disease that can occur in chronic infections or inflammatory diseases and is characterized by fibrillar deposition of serum amyloid A, an acute phase protein. Frequent renal involvement is manifested by proteinuria and renal insufficiency. In addition to treating the underlying disease, a new therapeutic target is the fibrillar depots. Eprodisate is a sulfonated molecule with structural similarity to heparan sulfate that binds to glycosaminoglycan-binding sites on serum amyloid A protein and inhibits fibril polymerization. A recent RCT showed a slowing of renal function decline [16].
Further studies need to confirm these results.
Conclusion for practice
- There is no general indication for dual RAAS blockade for the treatment of proteinuria.
- Rituximab is approved for induction therapy of ANCA-associated vasculitides and is used off-label for lupus nephritis and idiopathic membranous glomerulopathy.
- Eculizumab is approved for the treatment of hemolytic uremic syndrome, and initial studies are underway for use in C3 glomerulopathies.
- The successful use of abatacept in primary focal segmental glomerulosclerosis and of adrenocorticotropic hormone in proteinuric glomerulopathies has recently been described.
- Interesting initial study results exist on the administration of topical steroids in IgA nephropathy.
Literature:
- Mancia G, et al: 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press 2014; 23(1): 3-16.
- Mann JF, et al: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372(9638): 547-553.
- Parving HH, et al: Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367(23): 2204-2213.
- Mani LY, et al: [Rationale and clinical evidence for the use of rituximab in glomerular diseases]. Rev Med Suisse 2011; 7(290): 819-824.
- Specks U, et al: Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013; 369(5): 417-427.
- Jones RB, et al: Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363(3): 211-220.
- Rovin BH, et al: Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012; 64(4): 1215-1226.
- Merrill JT, et al: Efficacy and safety of rituximab in moderately-to-severely active systemic lupus eryth.: the randomized, double-blind, phase II/III SLE evaluation of rituximab trial. Arthritis Rheum 2010; 62(1): 222-233.
- Beck LH, et al: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361(1): 11-21.
- Ruggenenti P, et al: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 2012; 23(8): 1416-1425.
- Dooley MA, et al: Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 2013; 22(1): 63-72.
- Fakhouri F, et al: C3 glomerulopathy: a new classification. Nat Rev Nephrol 2010; 6(8): 494-499.
- Yu CC, et al: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 2013; 369(25): 2416-2423.
- Smerud HK, et al: New treatment for IgA nephropathy: enteric budesonide targeted to the ileocecal region ameliorates proteinuria. Nephrol Dial Transplant 2011; 26(10): 3237-3242.
- Ponticelli C, et al: A randomized pilot trial comparing ethylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 2006; 47(2): 233-240.
- Dember LM, et al: Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med 2007; 356(23): 2349-2360.
CARDIOVASC 2015; 14(1): 9-12