At the ASCO Congress, updates on immunotherapy and kinase inhibitors in melanoma were among the topics presented. Current research is yielding some promising insights into new treatment methods, for example with T-Vec or the antibodies anti-PD-1 and anti-PD-L1.
Antoni Ribas, MD, Los Angeles, gave an introductory talk on immunotherapy. A key treatment for melanoma was presented at ASCO, he said: The cancer-killing virus talimogene laherparepvec (usually just called T-Vec) is currently being tested in treatment. A randomized phase III study [1] showed a statistically significant improvement in the durable response rate (DRR), defined as objective response over at least six months, for oncolytic immunotherapy with T-Vec compared to granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with advanced stage IIIB-IV skin cancer. These results were obtained under a tolerable safety profile. DRR was set as the primary endpoint, but an interim analysis also showed a trend toward improved survival (important secondary endpoint). T-Vec thus shows great potential as a treatment option against melanomas especially with local metastases, but also against those with minimally disseminated distant metastases.
A randomized phase II study [2] also showed that addition of GM-CSF to the oncology drug ipilimumab significantly improved the primary endpoint, survival, compared with ipilimumab alone. No significant differences in toxicity were observed.
Lastly, Dr. Ribas presented new findings on anti-PD-1 and anti-PD-L1 antibodies, “They have significantly raised the bar for antitumor activity of immunomodulatory antibodies and represent the most promising agents for the treatment of melanoma. Combining anti-PD-1 and anti-PD-L1 antibodies may even improve the benefit in the future.”
Kinase inhibitors
Georgina Long, MD, Sydney, gave a presentation on kinase inhibitors. The so-called class I BRAF inhibitors show partly good results: Vemurafenib led to an increase in overall and progression-free survival in patients with previously untreated melanoma with the BRAF V600E mutation in a phase III study [3]. Similar results, particularly improvement in progression-free survival compared with treatment with dacarbazine, were shown in a randomized, controlled phase III trial of dabrafenib [4].
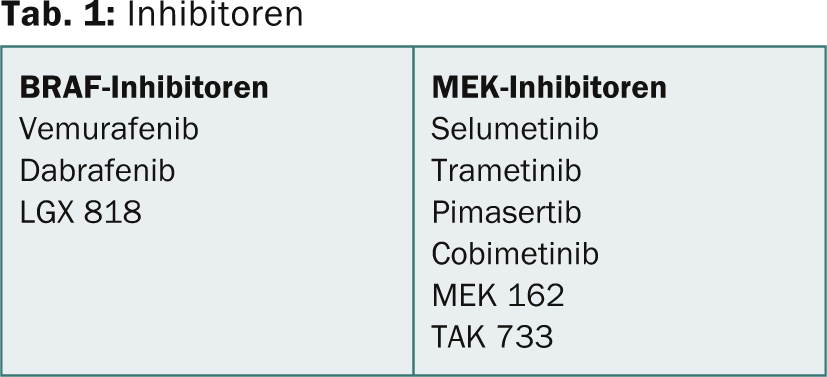
Further results are available on the combination of BRAF inhibition with MEK inhibition (Table 1). A 2012 study [5] demonstrated that dabrafenib and trametinib can be safely combined at full monotherapy doses. Although the risk of fever was increased with combination therapy, progression-free survival was significantly better.
Results from phase III trials [6] are also found for MEK inhibition alone, suggesting an improvement in overall and progression-free survival rates in patients with metastatic melanoma with BRAF V600E or V600K mutation. This compared with chemotherapy.
Possible other combinations and inhibitions include the following, according to Dr. Long:
- PI3K/AKT/mTOR inhibition
- MET Inhibition
- Cyclin D kinase 4/6 inhibition
- Multi-kinase inhibition.
Overall, according to Dr. Long, a combination of different drugs is useful, but the corresponding results from the current study situation must always be compared and critically examined in each case.
BRAF inhibitors
Keith T. Flaherty, MD, Boston, presented some complementary research on BRAF inhibitors: Sosman and colleagues [7] showed in a long follow-up that the median overall survival was about 16 months. Furthermore, the use of BRAF inhibitors in patients with metastatic melanoma appears to increase melanoma antigen expression, promote T-cell cytotoxicity, and provide a more favorable tumor microenvironment, arguing for the potential synergy of BRAF-targeted with immunotherapy [8].
Source: Annual Meeting of the American Society of Clinical Oncology (ASCO), May 31-June 4, 2013, Chicago.
- Andtbacka RHI, et al: OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol 2013; 31 (suppl; abstr LBA9008).
- Hodi FS, et al: Multicenter, randomized phase II trial of GM-CSF (GM) plus ipilimumab (Ipi) versus Ipi alone in metastatic melanoma: E1608. J Clin Oncol 2013; 31 (suppl; abstr CRA9007).
- Chapman PB, et al: Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med 2011; 364: 2507-2516 June 30, 2011DOI: 10.1056/NEJMoa1103782.
- Hauschild A, et al: Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012 Jul 28; 380(9839): 358-65. doi: 10.1016/S0140-6736(12)60868-X. Epub 2012 Jun 25.
- Flaherty KT, et al: Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012 Nov; 367(18): 1694-1703. doi: 10.1056/NEJMoa1210093. Epub 2012 Sep 29.
- Flaherty KT, et al: Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N Engl J Med 2012; 367: 107-114July 12, 2012DOI: 10.1056/NEJMoa1203421.
- Sosman JA, et al: Survival in BRAF V600-Mutant Advanced Melanoma Treated with Vemurafenib. N Engl J Med 2012; 366: 707-714 February 23, 2012DOI: 10.1056/NEJMoa1112302.
- Frederick DT, et al: BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clinical Caner Research. Published OnlineFirst January 10, 2013; doi: 10.1158/1078-0432.CCR-12-1630.
InFo Oncology & Hematology 2013; 1(1): 46-47.