Gene therapy, a long-time beacon of hope in medical research, is finally finding its way into the clinic to cure monogenetic diseases. This provides medicine with a new therapy that can treat hereditary diseases not only symptomatically, but also causally. Another gene therapy approach is the treatment of acquired gene alterations in malignancies using CAR(chimeric antigen receptor) T cells.
Gene therapy, a long-time beacon of hope in medical research, is finally finding its way into the clinic to cure monogenetic diseases. This provides medicine with a new therapy that can treat hereditary diseases not only symptomatically, but also causally. Another gene therapy approach is the treatment of acquired gene alterations in malignancies using CAR (chimeric antigen receptor) T cells. The following is an overview of the basic molecular biology and clinical application of commonly used gene and cell therapy techniques.
Genes as blueprints of all proteins – mutations as cause of monogenetic diseases
Every human cell contains a genetic blueprint inside the nucleus in the form of deoxyribonucleic acid (DNA). DNA consists of four basic building blocks, the bases adenine (A), thymine (T), guanine (G) and cytosine (C), which, lined up in long, linear chains, form the basic structure of the chromosomes of a human cell. The DNA of each cell contains individual coding units, the genes. Each gene consists of a coding region and a regulatory unit, the promoter, whose activity can be enhanced by an enhancer element if necessary. Each gene encodes the structure of a protein by the sequence of the four basic building blocks of DNA. Proteins are composed of 20 basic building blocks, amino acids, which fold together in one or more linear chains to form proteins. Only properly folded proteins can perform their biological function in the cell.
Monogenetic diseases
If faulty repair occurs after damage to the DNA, the sequence of the basic DNA building blocks can be altered. Alternatively, basic DNA building blocks can also be lost. These changes are called “mutations. If a mutation occurs in a section of DNA that codes for a protein, the structure of the protein encoded therein can be changed as a result: The defective protein synthesized according to the DNA blueprint cannot fulfill its natural function in the cell, or can only do so incompletely. This genetic change, in the simplest case e.g. an exchange of an A for a C within the DNA, can be the causal cause of a genetic disease, if the function of the cell in a tissue is reduced or even fails as a result, e.g.
- when the metabolism in the cells functions insufficiently and metabolites accumulate (e.g. lipoprotein lipase defect)
- when the synthesis of individual basic building blocks by metabolism is deficient (e.g. glucose-6-phosphate dehydrogenase defect)
- When individual components of the immune system are not functioning (e.g. Septic Granulomatosis or Severe Combined Immunodeficiency).
In a recent study, 4166 rare monogenetic diseases could be causally linked to 3163 genes [1]. Cure of the disease with genetic cause is possible only if it is possible to compensate or correct the causal cause.
New approaches to healing through gene therapy
Once a clearly defined gene mutation has been identified as the cause of a disease, that disease becomes amenable to potential gene therapy. Various methods are available:
- In diseases where the gene mutation results in loss of protein expression or expression of a defective protein (“loss-of-function” or “dysfunction” mutations), gene addition can be performed.
- In diseases where the gene mutation leads to overfunction of the protein (“gain-of-function” mutations), gene repair can be pursued.
Gene addition involves adding a gene to the cell’s genome that correctly encodes the missing or malfunctioning protein, thereby compensating for the function of the mutated gene. This results in a restoration of the protein’s original function. Gene addition has been tested in clinical trials for the treatment of numerous diseases, with clinical success for over 20 congenital hereditary diseases. All currently approved gene therapy products are based on gene addition.
Gene repair with nucleases such as CRISPR-Cas9 (fusion of nuclease Cas9 with DNA-binding CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats) restores the correct DNA sequence in the cell by targeting the mutation and correcting it in situ (principle: cut out and replace). Thus, the intended function of the protein is restored and subsequently there is a normalization of biological function. The use of gene repair (“gene scissors”/CRISPR-Cas) is possible regardless of whether “gain-of-function” or “loss-of-function” mutations are present. Such sequence-specific modification of genetic information is possible in the laboratory, but has so far only been used in oncology in the context of a few clinical studies.
Genaddition
First clinical successes by gene therapy were achieved in the field of immunology. Causes of congenital immunodeficiencies lie in the hematopoietic stem cells. Affected patients can therefore often be cured by transplantation of hematopoietic stem cells from an unrelated or family donor (allogeneic transplantation). Family members are rarely used as HLA-haploidentical donors because of higher rates of side effects. Family members who have higher HLA identity but also carry the genetic defect are more likely to be ineligible. In the absence of an HLA-identical donor (in Caucasians in about 1/3 of cases, in other ethnicities more frequently) autologous gene therapy can lead to cure of the disease.
For ex vivo gene therapy, the autologous cells are apherized after mobilization with G-CSF, purified, enriched and cultured ex vivo for a short period of time. During this period, the stem cells are treated with the gene therapy vector and then reinfused into the patient, usually after chemotherapy to ablate the bone marrow. Surprisingly, depending on the immunodeficiency, partial correction may be sufficient for significant clinical improvement.
The first publication on successful gene therapy appeared in 2000 on the treatment of infants with the most severe form of congenital immunodeficiency, Severe Combined Immunodeficiency, which usually leads to death without transplantation in the first year of life due to the most severe infections [2].
Between 2000 and 2006, all clinical successes in the field of gene therapy were achieved with the help of so-called retroviral vectors. These introduce the correction sequence in the form of RNA into the stem cells, where it is converted into DNA by reverse transcription and subsequently integrated into the patient’s DNA (gene addition). Subsequently, functional protein is formed and thus the defect is clinically corrected.
For in vivo gene addition, body cells are not removed, treated and returned, but viral particles with correction sequence are injected directly into the body. The first in vivo gene addition was published in 2007: for the treatment of Parkinson’s disease, patients were injected unilaterally, subthalamically with altered adeno-associated viral (AAV) particles encoding glutamic acid decarboxylase [3]. The vast majority of in vivo gene therapy clinical trials use modified AAV particles to deliver genetic information into the body. Exceptions include the use of modified herpes simplex virus-1(HSV-1)-derived viral particles [4] or non-replicating adenoviral particles [5] for the treatment of glioblastoma and the testing of HIV-1-derived viral particles for the in vivo treatment of Parkinson’s disease [6].
Side effects in first gene therapy studies
In the first generation of ex vivo gene therapieswith γ-retroviral vectors, the site of integration of the gene therapy vector in the genome of the target cell has been shown to play a key role with respect to potential side effects. These first-generation vectors preferentially integrated near transcription start sites. In first-generation γ-retroviral gene therapy vectors, protein expression was driven by a promoter in the gene therapy vector and enhanced by an enhancer. The enhancer of the first generation gene therapy vector was able to interact with the transcription start site of the gene in which the gene therapy vector was integrated. If integration into an oncogene took place, this oncogene could be activated in addition to therapeutic protein expression leading to cure of the underlying disease. As a result, stem cells in which this constellation occurred had acquired a growth advantage and were able to proliferate clonally. This resulted in some patients developing hematologic malignancies as a side effect of gene therapy in some clinical trials in various immunodeficiencies [7–10]. These side effects led to a switch from γ-retroviral vectors to HIV-1 virus-derived lentiviral vectors starting in 2009, as their integration profile promises more safety [11]. Furthermore, in so-called lentiviral self-inactivating (SIN) vectors, the HIV-1 virus-derived promoter and its enhancer element were removed. The absence of enhancer elements in second- and third-generation gene therapy vectors prevents enhancer-mediated transactivation of oncogenes after ex vivo treatment ofstem cells. To date, clinical trials have treated stem cells from an estimated close to 100 patients with lentiviral SIN vectors. To date, none of these clinical trials have resulted in the development of hematologic malignancies, indicating a significant improvement in safety compared to the first-generation vector.
The AAV-based gene therapy vectors used for in vivo gene additionintegrate into the genome of target cells only to a small extent. Therefore, insertional mutagenesis is unlikely. The greatest potential risk of AAV-based gene therapy vectors lies in pre-existing immunity, if any, to the surface structures of the AAV vector. In a clinical trial for the treatment of hemophilia B, a coagulation factor-IX-encoding AAV2 vector was used intramuscularly. A T-cell-mediated immune response resulted in the elimination of all genetically modified cells and terminated the therapeutic effect [12]. In contrast, intravenous administration resulted in uptake of the vectors into hepatocytes and long-term therapeutic effects in patients in whom antibodies to AAV could not be detected before inclusion in the study [13].
Gene therapy successes
The first generation of γ-retroviral gene therapy vectors provided evidence that gene therapy can lead to clinical success for X-SCID (X-linked severe combined immunodeficiency) [2], ADA (adenosine deaminase)-SCID [14], X-CGD (X-linked septic granulomatosis) [9], epidermolysis bullosa [15] and for Wiskott Aldrich syndrome (WAS) [16].
Due to the above-mentioned side effects of treating X-SCID, X-CGD, and WAS with first-generation gene therapy vectors (γ-retroviral vectors with full promoter/enhancer), lentiviral SIN vectors were developed, leading to a clinical success story. To date, lentiviral SIN vectors have achieved clinical success in ex vivo gene therapy in the treatment of
- X-linked adrenoleukodystrophy (ALD) [17]
- γ-Thalassemia [18,19]
- WHAT [20]
- X-SCID [21]
- ADA-SCID [22]
- Sickle cell disease [23]
- COL7A1 Epidermolysis Bullosa [24]
- Metachromatic leukodystrophy [25]
Meanwhile, AAV-mediated in vivo gene therapyhas also been used in the treatment of at least 14 indications:
Neurology
- Parkinson’s [3,26,27]
Ophthalmology
- Liver’s cong. Amaurose [28,29]
- Chorioideremia [30,31]
- Age-related macular degeneration [32]
- Leber’s hereditary optic neuropathy [33,34]
Hematology
- Hemophilia B [13]
Muscular dystrophy
- Becker muscular dystrophy [35]
- Spinal Muscular Atrophy Type I (SMA1) [36]
Metabolic diseases
- Pompe disease [37]
- α-1-antitrypsin (AAT) deficiency [38,39]
- Mucopolysaccharidosis type IIIB [40]
- Aromatic amino acid decarboxylase deficiency [41,43]
- Lipoprotein lipase deficiency [42]
In the treatment of leukemia, CAR-T cells are used in numerous studies and now also approved therapies. For this purpose, chimeric antigen receptors are introduced into autologous T cells from affected patients. This enables these genetically modified T cells (CAR-T cells) to recognize and eliminate tumor cells [44–46].
Genre Repair
To date, it has not been possible to have lenti- or γ-retroviral gene therapy vectors integrated into predetermined locations in the genome. Sequence-specific insertion and/or correction became possible only after it was possible to cut the DNA in a sequence-specific manner. This is done using nucleases such as CRISPR-Cas. In this gene therapy system, a fusion of the DNA-binding Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and the DNA-cutting Cas9 (CRISPR/Cas9) [47] has been produced. The resulting break in the DNA can then be repaired by the cell in two different ways: Either the ends are reconnected in a defective process called non-homologous end joining. The second repair mechanism is homologous recombination. This pathway is taken when there is repair DNA in the cell whose ends match the DNA sequence at the site of the DNA break. This repair DNA is administered to the cells together with the nuclease and serves as a genetic correction template. In homologous recombination, the DNA break is then closed by the endogenous cellular repair enzymes according to the DNA sequence of the repair DNA. This makes it possible to introduce a correction gene into the genome in a sequence-specific manner or to correct point mutations genetically. Sequence-specific gene addition has not yet found clinical application because the efficiency of the method has been limiting so far. However, recent technical developments bring clinical trials within the realm of possibility.
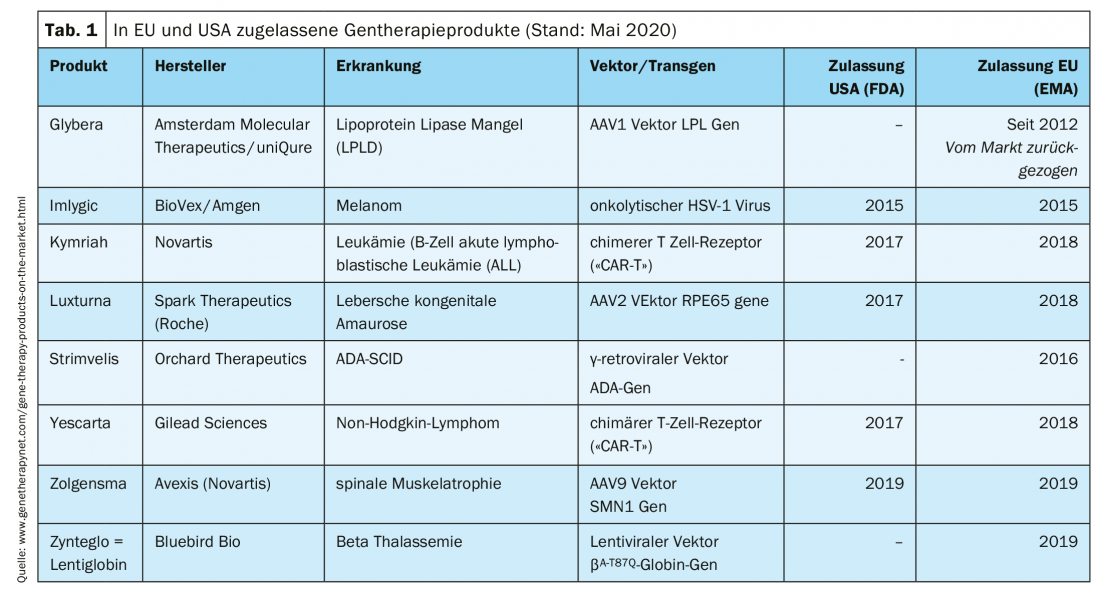
Clinically approved gene therapy products
Treatment of patients with gene therapy products is possible in specialized centers. Several products have already received marketing authorization (Tab. 1).
Take-Home Messages
- Gene therapy as a causal treatment for monogenetic inherited diseases is increasingly finding its way into the clinic.
- Using viral vectors, gene therapy based on gene addition has been successfully used in clinical trials since 2000 for certain hematologic
- diseases and congenital immunodeficiencies.
- Targeted gene repair using nucleases has been used in few studies for oncological diseases where the function of a gene is to be knocked down.
- Some malignancies can be treated using genetically engineered T cells (CAR-T cells).
- Gene therapeutics are currently already available as approved products for 7 diseases.
Literature:
- Ehrhart F, et al: History of rare diseases and their genetic causes – a data driven approach. bioRxiv 2020; preprin doi: https://doi.org/10.1101/595819
- Cavazzana-Calvo, M et al: Gene Therapy of Human Severe Combined Immunodeficiency (SCID)-X1 Disease. Science 2000; 288: 669-672.
- Kaplitt MG, et al: Safety and Tolerability of Gene Therapy With an Adeno-Associated Virus (AAV) Borne GAD Gene for Parkinson’s Disease: An Open Label, Phase I Trial. Lancet 2007; 369(9579): 2097-2105.
- Todo T: Active Immunotherapy: Oncolytic Virus Therapy Using HSV-1 Adv Exp Med Biol. 2012; 746: 178-186.
- Brenner AJ, et al: Safety and Efficacy of VB-111, an Anticancer Gene Therapy, in Patients With Recurrent Glioblastoma: Results of a Phase I/II Study. Neuro Oncol 2020; 22(5): 694-704.
- Palfi S, et al: Long-Term Follow-Up of a Phase I/II Study of ProSavin, a Lentiviral Vector Gene Therapy for Parkinson’s Disease. Hum Gene Ther Clin Dev 2018; 29(3): 148-155.
- Hacein-Bey-Abina S, et al: Serious Adverse Event After Successful Gene Therapy for X-linked Severe Combined Immunodeficiency. N Engl J Med 2003; 348(3): 255-256.
- Aiuti A, et al: The Committee for Advanced Therapies’ of the European Medicines Agency Reflection Paper on Management of Clinical Risks Deriving from Insertional Mutagenesis. Human Gene Ther Clin Dev 2013; 24: 47-54.
- Ott MG, et al: Correction of X-linked Chronic Granulomatous Disease by Gene Therapy, Augmented by Insertional Activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 2006; 12(4): 401-409.
- Siler U, et al: Successful Combination of Sequential Gene Therapy and Rescue Allo-HSCT in Two Children With X-CGD – Importance of Timing. Curr Gene Ther 2015; 15(4): 416-427.
- Serrao E, et al: Sites of Retroviral DNA Integration: From Basic Research to Clinical Applications. AN Crit Rev Biochem Mol Biol 2015; 28: 1-17.
- Manno CS, et al: Successful Transduction of Liver in Hemophilia by AAV-Factor IX and Limitations Imposed by the Host Immune Response. Nat Med 2006; 12: 342-347.
- Nathwani AC, et al: Long-term Safety and Efficacy of Factor IX Gene Therapy in Hemophilia B. N Engl J Med 2014; 371(21): 1994-2004.
- Aiuti A, et al: Correction of ADA-SCID by Stem Cell Gene Therapy Combined With Nonmyeloablative Conditioning. Science 2002; 296(5577): 2410-2413.
- Mavilio F, et al: Correction of Junctional Epidermolysis Bullosa by Transplantation of Genetically Modified Epidermal Stem Cells. Nat Med 2006; 12(12): 1397-1402.
- Boztug K, et al: Stem-cell Gene Therapy for the Wiskott-Aldrich Syndrome. N Engl J Med 2010; 363(20): 1918-1927.
- Cartier N, et al: Hematopoietic Stem Cell Gene Therapy With a Lentiviral Vector in X-linked Adrenoleukodystrophy. Science 2009; 326(5954): 818-823.
- Cavazzana-Calvo M, et al: Transfusion Independence and HMGA2 Activation After Gene Therapy of Human β-Thalassaemia. Nature 2010; 467(7313): 318-322.
- Thompson AA, et al: Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. N Engl J Med 2018; 378: 147993.
- Aiuti A, et al: Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 2013; 341(6148): 1233151.
- De Ravin SS, et al: Lentiviral Hematopoietic Stem Cell Gene Therapy for X-linked Severe Combined Immunodeficiency. Sci Transl Med 2016; 8(335): 335ra57.
- Mullard A: EMA greenlights second gene therapy. Nat Rev Drug Discov 2016; 15: 299.
- Ribeil JA, et al: Gene Therapy in a Patient With Sickle Cell Disease. N Engl J Med 2017; 376(9): 848-855.
- Lwin SM, et al: Safety and Early Efficacy Outcomes for Lentiviral Fibroblast Gene Therapy in Recessive Dystrophic Epidermolysis Bullosa. JCI Insight 2019; 4(11): e126243.
- Biffi A, et al: Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013; 341(6148): 1233158.
- Niethammer M, et al: Gene Therapy Reduces Parkinson’s Disease Symptoms by Reorganizing Functional Brain Connectivity. Sci Transl Med 2018; 10(469): eaau0713.
- Heiss JD, et al: Trial of Magnetic Resonance-Guided Putaminal Gene Therapy for Advanced Parkinson’s Disease. Mov Disord 2019; 34(7): 1073-1078.
- Bainbridge JWB, et al: Effect of Gene Therapy on Visual Function in Leber’s Congenital Amaurosis. N Engl J Med 2008; 358(21): 2231-2239.
- Bainbridge JWB, et al: Long-term Effect of Gene Therapy on Leber’s Congenital Amaurosis. N Engl J Med 2015; 372(20): 1887-1897.
- MacLaren RE, et al: Retinal Gene Therapy in Patients With Choroideremia: Initial Findings From a Phase 1/2 Clinical Trial. Lancet 2014; 383: 1129-1137.
- Xue K, et al: Beneficial Effects on Vision in Patients Undergoing Retinal Gene Therapy for Choroideremia. Nat Med 2018; 24(10): 1507-1512.
- Rakoczy EP, et al: Gene Therapy With Recombinant Adeno-Associated Vectors for Neovascular Age-Related Macular Degeneration: 1 Year Follow-Up of a Phase 1 Randomised Clinical Trial. Lancet 2015; 386(10011): 2395-2403.
- Feuer WJ, et al: Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results. Ophthalmology 2015; 123(3): 558-570.
- Bouquet C, et al: Immune Response and Intraocular Inflammation in Patients With Leber Hereditary Optic Neuropathy Treated With Intravitreal Injection of Recombinant Adeno-Associated Virus 2 Carrying the ND4 Gene: A Secondary Analysis of a Phase 1/2 Clinical Trial. JAMA Ophthalmol 2019; 137(4): 399-406.
- Mendell JR, et al: A Phase 1/2a Follistatin Gene Therapy Trial for Becker Muscular Dystrophy. Mol Ther 2015; 23(1): 192-201.
- Mendell JR, et al: Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017; 377(18): 1713-1722.
- Corti M, et al: B-Cell Depletion Is Protective Against Anti-AAV Capsid Immune Response: A Human Subject Case Study. Mol Ther Methods Clin Dev 2014; 1: 14033.
- Calcedo R, et al: Class I-restricted T-cell Responses to a Polymorphic Peptide in a Gene Therapy Clinical Trial for α-1-antitrypsin Deficiency. Proc Natl Acad Sci U S A 2017; 114(7): 1655-1659.
- Mueller C, et al: Year Expression and Neutrophil Defect Repair After Gene Therapy in Alpha-1 Antitrypsin Deficiency. Mol Ther 2017; 25(6): 1387-1394.
- Tardieu M, et al: Intracerebral Gene Therapy in Children With Mucopolysaccharidosis Type IIIB Syndrome: An Uncontrolled Phase 1/2 Clinical Trial. Lancet Neurol 2017; 16(9): 712-720.
- Chien YH, et al: Efficacy and Safety of AAV2 Gene Therapy in Children With Aromatic L-amino Acid Decarboxylase Deficiency: An Open-Label, Phase 1/2 Trial. Lancet Child Adolesc Health 2017; 1(4): 265-273.
- Kassner U, et al: Gene Therapy in Lipoprotein Lipase Deficiency: Case Report on the First Patient Treated With Alipogene Tiparvovec Under Daily Practice Conditions. Hum Gene Ther 2018; 29(4): 520-527.
- Kojima K, et al: Gene Therapy Improves Motor and Mental Function of Aromatic L-Amino Acid Decarboxylase Deficiency. Brain 2019; 142(2): 322-333.
- Porter DL, et al: Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med 2011; 365(8): 725-733.
- Savoldo B, et al: CD28 Costimulation Improves Expansion and Persistence of Chimeric Antigen Receptor-Modified T Cells in Lymphoma Patients. J Clin Invest 2011; 121(5): 1822-1826.
- Grupp SA, et al: Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med 2013; 368(16): 1509-1518.
- Yang G, Huang X: Methods and Applications of CRISPR/Cas System for Genome Editing in Stem Cells. Cell Regen (Lond) 2019; 8(2): 33-41.
FAMILY PRACTICE 2020; 15(9): 6-10