At the end of March, more than 13,000 cardiologists from around the world gathered in Washington DC to present and discuss the latest scientific findings in the field of prevention, diagnosis and treatment of cardiovascular disease at the annual meeting of the American College of Cardiology (ACC).
Scientific highlights of this congress included important topics in the field of invasive therapy of cardiovascular diseases and included catheter-based therapy of severe aortic valve stenosis (TAVI), endovascular treatment of refractory arterial hypertension, and the use of direct thrombin antagonists during primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Below, we summarize key trial findings from the “CoreValve U.S. Pivotal High-Risk” trial, the “Simplicity HTN-3” Investigation, and the HEAT-PPCI(“How Effective are Antithrombotic Therapies in PPCI”) trial.
Transcatheter Aortic Valve Implantation with the CoreValve Bioprosthesis
TAVI has been the established treatment for inoperable patients with symptomatic, severe aortic valve stenosis since demonstrating a significant survival benefit in a randomized comparison with medical therapy alone (mortality in PARTNER B: TAVI 30.7% versus 50.7% with medical therapy at one year, p<0.001). Furthermore, the first randomized comparison between conventional surgical aortic valve replacement and TAVI (PARTNER A) demonstrated an equivalent outcome in selected high-risk patients (mortality in PARTNER A: TAVI 24.2% versus 26.8% in surgery at one year, p=0.44 [ITT]). While this comparison was performed with the first generation balloon-expandable Edwards Sapien prosthesis, no comparable data were previously available with the self-expandable Medtronic CoreValve bioprosthesis. This made the results of the “CoreValve U.S. Pivotal” study in selected patients at increased risk, which were published in the New England Journal of Medicine at the same time as the presentation, all the more anticipated. In a direct randomized comparison between TAVI with the self-expandable CoreValve prosthesis and surgical aortic valve replacement, 795 patients were included. With a mean anticipated mortality risk of 7.4%, according to the STS risk score, the clinical characteristics of the two groups of patients were balanced: 75% of patients had concomitant coronary artery disease, 13% of patients had a previous stroke, and 25% of patients had a myocardial infarction in their cardiac history. All were assessed as high-risk patients by a local heart team and presented with NYHA class III or IV in more than 85% of cases. At 30 days, a mortality rate of 3.3 after TAVI and 4.5% after open aortic valve replacement was observed, which was significantly lower than anticipated. After one year, catheter therapy with the CoreValve bioprosthesis was significantly superior to surgery in the primary endpoint of mortality (14.2 vs. 19.1%, p=0.04) (Fig. 1) .
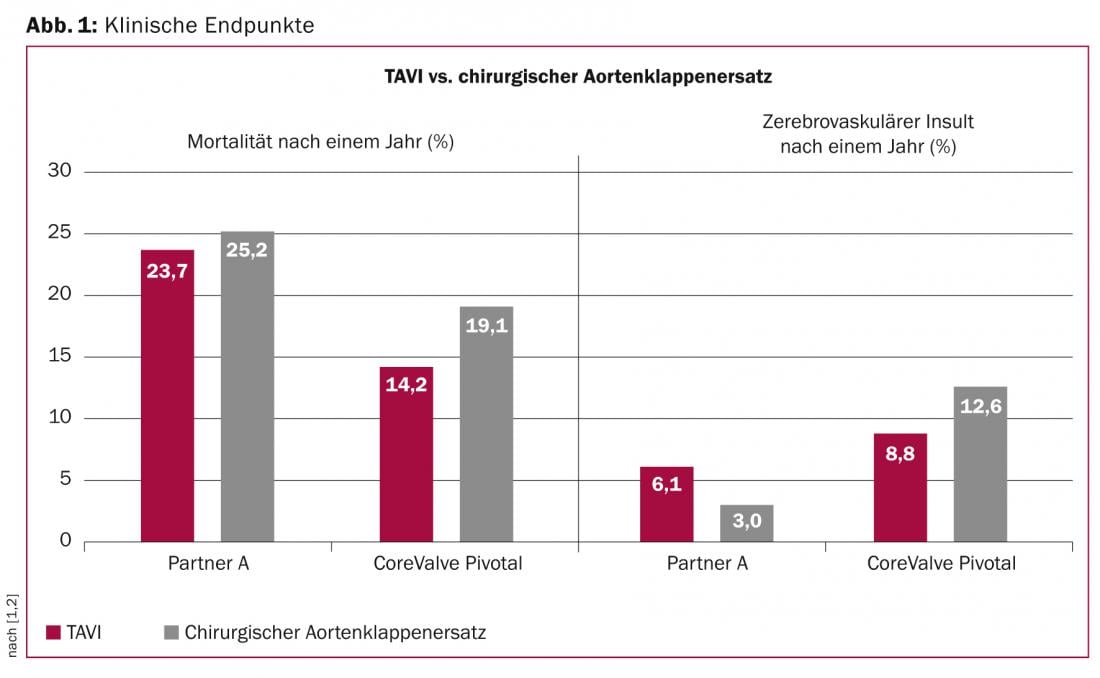
Life-threatening bleeding complications (13.6 vs. 35.0%, p<0.001), acute worsening of renal function (6.0 vs. 15.1%, p<0.001) and new-onset atrial fibrillation (11.7 vs. 30.5%, p<0.001) were less common in TAVI patients within the first 30 days, while vascular complications (5.9 vs. 1.7%, p=0.003) and pacemaker implantations (19.8 vs. 7.1%, p<0.001) were observed to be increased. With regard to the incidence of cerebral strokes, there were no differences between the two forms of therapy either after 30 days (4.9 vs. 6.2%, p=0.46) or after twelve months (8.8 vs. 12.6%, p=0.10). Whereas the mean transvalvular gradient was lower (9± 4 mmHg vs. 12±7 mmHg, p<0.001) and aortic valve opening area (1.91±0.5 cm2 vs. 1.57±0.5 cm2, p<0.001) were significantly greater one year after TAVI than after surgical aortic valve replacement, moderate or severe paravalvular aortic regurgitation was more common after TAVI (6.1%) compared with surgical aortic valve replacement (0.5%, p<0,001).
In conclusion, this study indicates for the first time a survival advantage of TAVI over surgical aortic valve replacement in selected patients at increased risk.
Renal Denervation – Simplicity HTN-3
Following the concept of surgical, non-selective sympathectomy and the associated therapeutic effect of lowering blood pressure, the successful catheter intervention with selective sympathetic elimination in the sense of renal denervation was presented by Dr. MP Schlaich in 2009. Promising results with sustained blood pressure reduction were shown in the first observational studies (Simplicity HTN-1 and HTN-2) in patients with refractory arterial hypertension. At ACC 2014, the results of the Simplicity HTN-3 study were presented and the results were published simultaneously with the congressional presentation in the New England Journal of Medicine. The Simplicity HTN-3 trial was a blinded, randomized study comparing catheter-based renal denervation with a sham procedure using renal artery angiography alone. A total number of 535 patients with refractory arterial hypertension, an ambulatory systolic blood pressure >160 mmHg, and at least three different antihypertensive medication classes were included in the study. Patients were treated with a mean of five different drug classes, four of which were already administered at maximum tolerated doses. Six months after randomization between renal denervation and sham procedure, a reduction in ambulatory blood pressure of 14.1±24 mmHg was demonstrated in both the denervation group and 11.7±26 mmHg in the control group. The mean difference of 2.4 mmHg (95%CI -6.9 to 2.1), however, showed no significant difference between renal denervation using the Simplicity catheter and the control procedure (p=0.26) and thus no measurable effect of the procedure on blood pressure six months after study inclusion (Fig. 2). The primary safety endpoint of the study-a combination of deaths, severe renal insufficiencies, embolic events, renovascular complications, and hypertensive crises-occurred with similar frequency in both groups (renal denervation 4.0 vs. control group 5.8%).
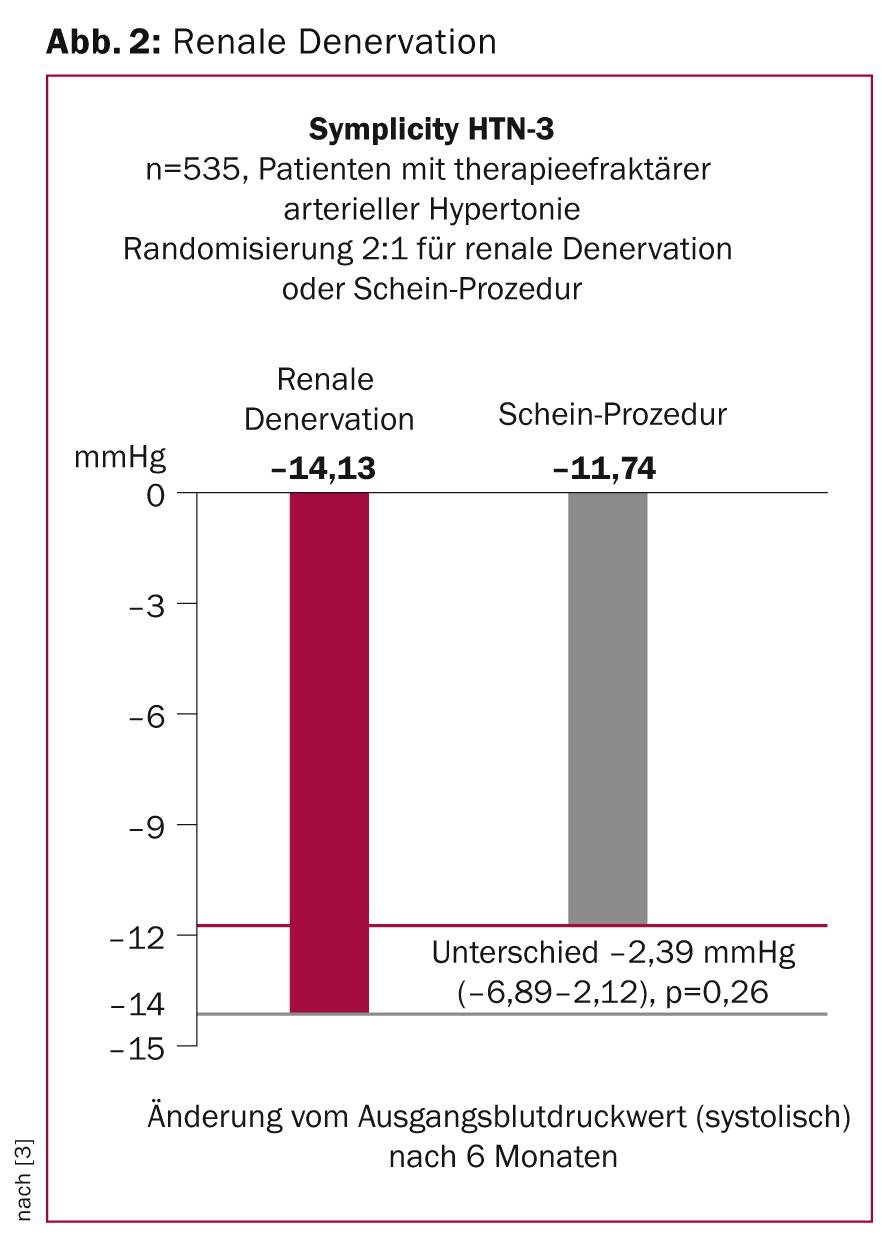
In light of the results of previous studies, the therapeutic concept of renal denervation will be re-evaluated after the negative results of this study. Limitations regarding the experience of the surgeons, technical details regarding the catheter used, the intensive drug therapy of the selected study population, and the lack of in vivo evidence of actual renal denervation require further investigation.
Bivalirudin vs. heparin during primary percutaneous coronary intervention.
Great attention was paid to the presentation of the HEAT-PPCI trial, which evaluated the safety and efficacy of peri-procedural administration of bivalirudin in direct comparison to heparin in primary percutaneous coronary intervention in 1829 patients with acute ST-segment elevation myocardial infarction. In the HORIZON AMI study published in 2008, bivalirudin was superior to unfractionated heparin in combination with glycoprotein IIb/IIIa antagonists in terms of major bleeding, but also cardiovascular events including death and myocardial infarction. In the meantime, practice has changed such that glycoprotein IIb/IIIa antagonists are used less frequently, new potent P2Y12 receptor antagonists (ticagrelor, prasugrel) are used systematically in acute myocardial infarction, and radial access is associated with fewer bleeding complications. With this in mind, the HEAT-PPCI trial compared unfractionated heparin at a standard dose of 70 IU/kgKG directly with bivalirudin in the form of a bolus of 0.75 mg/kgKG followed by infusion. Glycoprotein IIb/IIIa antagonists were allowed as “bail-out” therapy in both groups (14%). More than 80% of patients were treated with a radial access route, mechanical thrombectomy was performed in close to 60%, 85% of patients received new P2Y12 receptor antagonists, and 80% of patients were treated with a drug-eluting stent. The primary end point was a composite end point of death, stroke, reinfarction, and unplanned revascularization at 1 month. In a direct comparison of the two therapeutic methods, the administration of unfractionated heparin during primary coronary intervention was shown to be superior to therapy with bivalirudin with respect to the primary endpoint (relative risk 1.52, 95%CI 1.1-2.1, p=0.01). Specifically, the end points of reinfarction (bivalirudin 2.7 vs. 0.9% heparin) and unplanned revascularizations (bivalirudin 2.7 vs. 0.7% heparin) were responsible for this difference. In addition to the primary endpoint, an increased number of stent thromboses (bivalirudin 3.4 vs. 0.9% heparin; RR 3.91, 95%CI 1.6-9.5, p=0.001) was recorded as in other studies with bivalirudin, whereas major bleeding complications were not different comparing the two treatment groups (bivalirudin 3.5 vs. 3.1% heparin; RR 1.15, 95%CI 0.7-1.9, p=0.59) (Fig. 3).
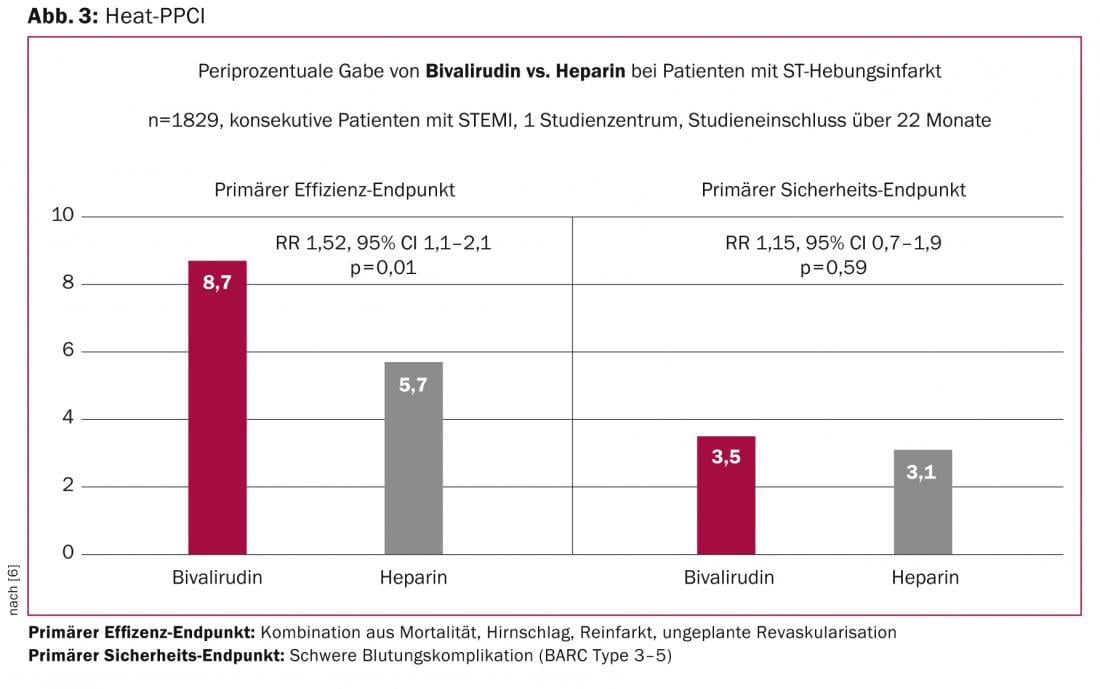
The lack of difference in the safety endpoint of relevant bleeding was unexpected, as previous comparative studies attributed greater safety to short-acting bivalirudin in preventing bleeding complications during or after PCI. The explanation for the lack of safety effect of bivalirudin compared with heparin therapy in this study might be found in the high rate of interventions via radial access (80% of interventions).
Prof. Dr. med. Stephan Windecker
Literature:
- Adams DH, et al: Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370(19): 1790-1798.
- Smith CR, et al: Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364(23): 2187-2198.
- Bhatt DL, et al: A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370(15): 1393-1401.
- Esler MD, et al: Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376(9756): 1903-1909.
- Stone GW, et al: Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008; 358(21): 2218-2230.
- Shahzad A, Stables R: HEAT PPCI – How effective are Antithrombotic Therapies in PPCI. Heparin versus bivalirudin in PPCI. presented at ACC 2014 in Washington, DC 2014.
CARDIOVASC 2014; 13(3): 14-17











