The first step in prescribing inhalation therapy is to select the optimal device. This must be based on patient factors (ability to cooperate, achievable inspiratory flow, and severity of obstruction) and knowledge of the physical and technical characteristics of the device. Good instruction and periodic review of inhalation therapy is important. In COPD (“Chronic Obstructive Pulmonary Disease”) with moderate to severe obstruction, the first choice for inhalation therapy is a combination preparation LAMA/LABA, which causes a functional improvement and reduction of the exacerbation rate. Topical steroids should be considered only when clearly indicated (asthma component) or with unsatisfactory results under LAMA/LABA at FEV1 <50% in the form of triple therapy. Basic therapy of bronchial asthma is a combination ICS/LABA with symptom-adjusted ICS dose. With Symbicort, therapy according to the SMART principle (“Symbicort Maintenance and Reliever Therapy”) is also possible.
For a long time, only a few drugs were available for the treatment of bronchial asthma and COPD, but in just a few years the number of substances, combinations and dosage forms has increased enormously. Especially in the therapy of COPD, such a rapid development has occurred that it is difficult for non-specialists to get an up-to-date overview.
We are used to thinking about medication selection, dosage and administration intervals, as well as any interactions. In the case of inhalation therapy, the optimal form of administration must also be selected. It is well known that adherence to inhaled therapies is often poor; application errors are often made, resulting in ineffective treatment [1]. This circumstance must be taken into account in the choice of therapy.
Influence of particle- and patient-dependent factors
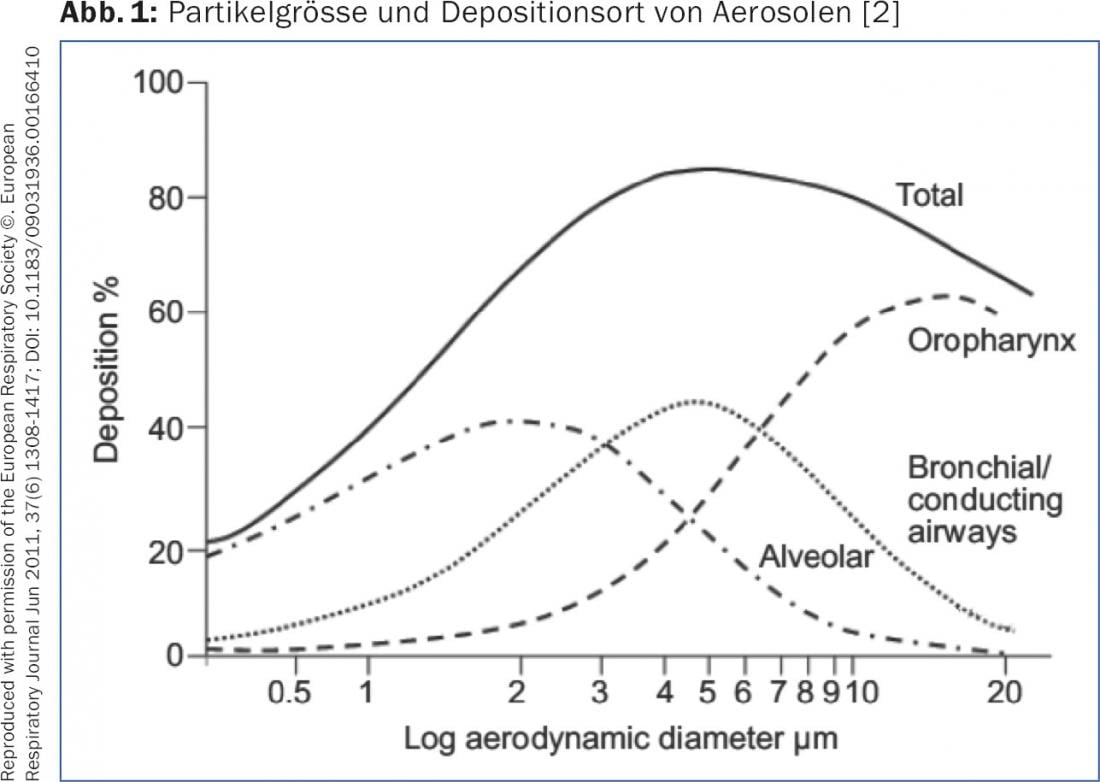
The deposition of an aerosol in the airways as well as the particles of a powder inhaler depends on the particle size (“Mass Median Aerodynamic Diameter” [MMAD]) and the inspiratory flow. Particles with an MMAD of >5 μm reach only the central airways and not, as desired, the peripheral airways. Particles with an MMAD of 1-5 μm, the so-called “Fine-Particle Fraction” (FPF), enter the middle and small airways – as desired for therapy with bronchodilators or topical steroids – and are deposited here by sedimentation. Very small particles with an MMAD of <1 μm reach the alveoli by diffusion or are exhaled again (Fig. 1). To ensure good sedimentation of the particles, at the end of inhalation, the breath should be held for 5-10 sec. be stopped. To optimize the deposition of a metered dose aerosol (DA) with or without an upstream chamber, the adult should be exposed over 4-5 sec. inhaled. This guarantees an optimum flow rate of approx. 30 l/min. With a powder inhaler, inhalation must be as deep and firm as possible to generate sufficient FPF. A turbulent flow must be created within the device, which disaggregates the particles and generates the desired fine particles [2,3]. Additionally, the presence of lung disease affects aerosol deposition. In cases of severe obstructive ventilation disorder or “mucus plugging”, significantly fewer particles are deposited peripherally. Bronchodilators develop their greatest effect in the middle airways (so-called “conducting airways”) while corticosteroids are probably better distributed uniformly to the periphery, since the inflammatory changes manifest themselves in the smallest airways.
For inhalation therapy to be effective, it must not only be performed regularly, but also technically correctly. Multiple studies have shown that only about 30% of patients use their metered dose inhaler or powder inhaler optimally, another about 40% use it acceptably, but about 30% use it with inadequate technique [4,5]. Good instruction and regular monitoring of inhalation technique are essential factors for successful inhalation therapy. Older patients in particular often do not use their inhalers correctly despite good instruction. The choice of inhalation therapy must thus also be based on the patient’s capabilities. If possible, different systems should not be prescribed at the same time. The prescription of combination preparations is useful to simplify the therapy. Once a patient has mastered the use of one inhaler, they should not be switched to another without good reason.
Inhalation devices available
Metered dose (DA) aerosols: DA have been the most popular route of administration since the 1950s. The container is pressurized, and the drug and propellant are in liquid form. The chlorofluorocarbon (CFC) formerly used as a propellant has been replaced by hydrofluoroalkanes (HFA) in all DA since the ban. Compared to the former CFC-DA, the discharge velocity of HFA-DA is somewhat lower, the cold sensation (so-called Freon effect) somewhat lower. Exit velocity and cooling effect can be further reduced by adding some alcohol, as is the case in Foradil®, Alvesco® and Qvar®. Most DAs are in dispersion form and must be shaken before use. Exceptions are the topical steroids Alvesco® (ciclesonide) and Qvar® (beclomethasone diproprionate), in which the drug is dissolved in the propellant. These DA produce much smaller particles of only 1.2 μm MMAD (“Extra-Fine Particles”) with better peripheral deposition at much lower exit velocity. For first use and non-use for several days, all DA should be prepared with spraying two strokes into the environment. Conventional DA should be used with an upstream chamber to overcome the problem of high exit velocity and difficult coordination. Pulmonary deposition can thus be increased from 15 to about 40%. DA with dissolved drug achieve a very good deposition of up to 60% even without an upstream chamber. Nevertheless, to minimize coordination problems, the use of an upstream chamber is often recommended. The former large upstream chambers have disappeared; today, only smaller, antistatic upstream chambers such as Aerochamber plus® or Vortex® are used. Ventolin DA as an on-the-go emergency medication can be used without a prechamber as a compromise, but not in the office or emergency department as for testing reversibility on spirometry.
Powder inhalers (“Dry Powder Inhaler” [DPI]): Several new DPIs have been introduced in recent years. All require an inspiration flow that varies from product to product to generate the particles [6]. However, this is always higher than with a DA. Most patients are able to generate a sufficient inspiratory flow. In severe hyperinflation, the ability to provide sufficient inspiratory volume to inhale the entire volume of medication is more of a problem. This can be compensated for in capsule systems by inhaling twice. Furthermore, the DPI differ in their inhalation resistance. Although high resistance makes it difficult to achieve a high flow, the resulting lower inhalation speed leads to a more homogeneous deposition. The Handyhaler® (for Spiriva) and Breezhaler® (for Onbrez®, Seebri®, Ultibro®) capsule systems are small and handy, but inserting the capsules requires some skill. The patient must be informed that the capsules – when properly inserted – develop a vibrating sound during inhalation. One advantage of these systems is that it is possible to check whether the patient has inhaled the entire amount of medication (open capsules in the Handyhaler®, transparent capsules in the Breezhaler®). In addition to the capsule systems, several multidose DPIs are available. The Turbuhaler® (for Symbicort®, Pulmicort®, Bricanyl) has been established for years, is handy and simple, but is somewhat prone to handling errors, especially in older patients. Turbu+® will soon be available in Switzerland, an electronic, app-based accessory that attaches to the Turbuhaler and enables seamless documentation of inhalation therapy via a smartphone. The intuitive Discus® (for Serevent®, Axotide®, Seretide®) is likely to be increasingly displaced by the even easier-to-use Ellipta® (for Anoro®, Relvar®, Incruse®, Arnuity®), which is automatically loaded when the cap is opened. The Gennuair® (for Eklira®) is the only DPI that guarantees sufficient inspiratory flow, in that the device does not release the dose until a sufficient inspiratory flow is reached. DPI achieve satisfactory pulmonary deposition of about 30%.
Soft Mist Inhaler: The Soft Mist Inhaler System Respimat® (for Spiolto®, Striverdi® and soon also Spiriva®), a handy multidose inhaler, has recently become available in Switzerland. With the aid of a spring mechanism, the drug solution is forced through two silicon nozzles and a very fine aerosol is produced. This is characterized by a low exit velocity and a very stable spray cloud (up to 1.5 sec.). A propellant gas is not necessary. The Respimat® enables relatively slow inhalation and makes fewer demands on coordination than the usual DA. Particle size, unlike DPI, is independent of inspiration flow. Very good pulmonary deposition of 40-50% of the released dose is achieved.
Moist inhalation: The use of a nebulizer device today should be limited to individual cases if a satisfactory result cannot be achieved with the devices listed above and to emergency situations in COPD and asthma. The particle size is determined by the nebulizer head and the applied flow. Only nebulizer heads and compressors that have been validated should be combined. When using compressed air from a wall connection, the optimum flow rate for the nebulizer head (usually 6-8 l/min) must be observed. Nebulizers with a valve system that prevents the loss of aerosol to the environment (e.g. Pari LC Plus) increase the available drug dose. Whenever possible, adults should inhale with a mouthpiece rather than a face mask to avoid the undesirable filtering function of the nose. For moist inhalation, only short-acting bronchodilators and a topical steroid (Pulmicort® Respules) are available.
Drug choice
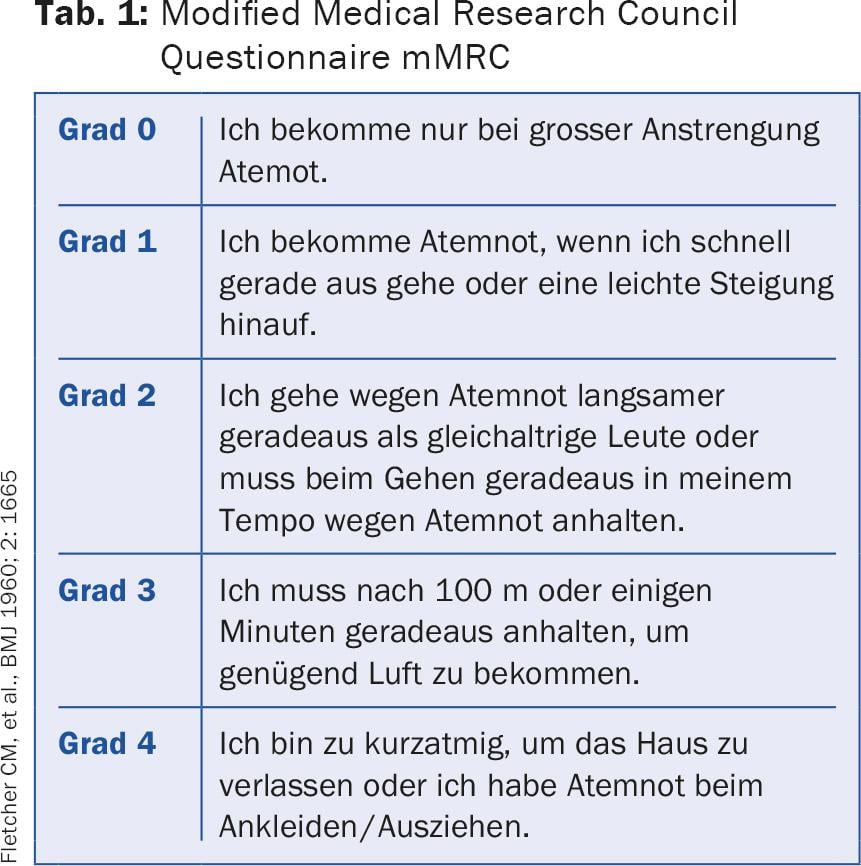
COPD: The goal of therapy in COPD is to improve lung function, but especially physical performance and quality of life. The reduction of the exacerbation rate is also essential. Exacerbations of COPD are a prognostically unfavorable factor [7]. They are the cause of costly hospitalizations, are very stressful for the patients and massively impair the quality of life. For this reason, current treatment recommendations are no longer based on the severity of obstruction alone, but on a multidimensional COPD classification, which is based on the severity of obstruction, dyspnea as measured by the simple modified Medical Research Council Questionnaire (mMRC) (Tab. 1) or the COPD Assessment Test (CAT), which uses eight questions to assess the symptoms of the disease [8]. The test results in a score of 0-40. The CAT questionnaire is available in many languages and can be accessed online [9]. The third determining factor is the frequency of exacerbations; up to one exacerbation/year without hospitalization is tolerable; patients with ≥2 exacerbations or one hospitalization are judged to have received unsatisfactory therapy. Based on these factors, COPD patients can be divided into four groups (A-D), which guide therapy (Fig. 2).
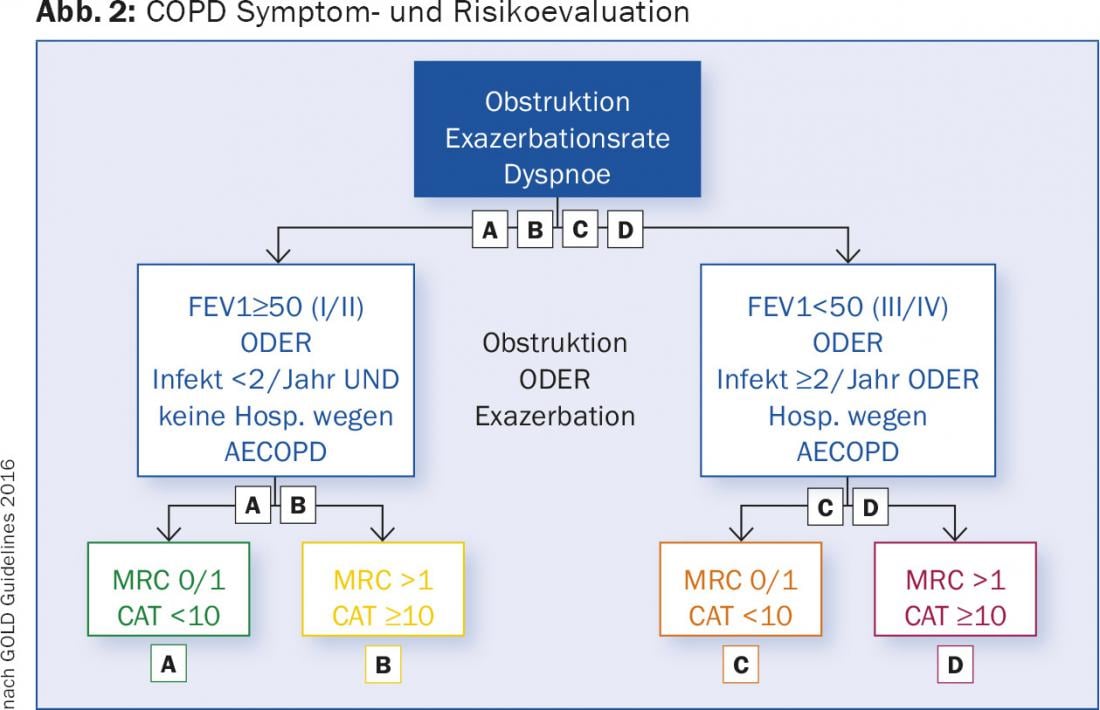
The treatment guidelines are presented in detail as well as in short form on the homepage of the “Global Initiative for Obstructive Lung Disease” [10]. The following drug classes are used: short-acting betamimetic (SABA), long-acting betamimetic (LABA), long-acting antimuscarinic (LAMA), and topical steroids (ICS) (Tables 2 and 3).
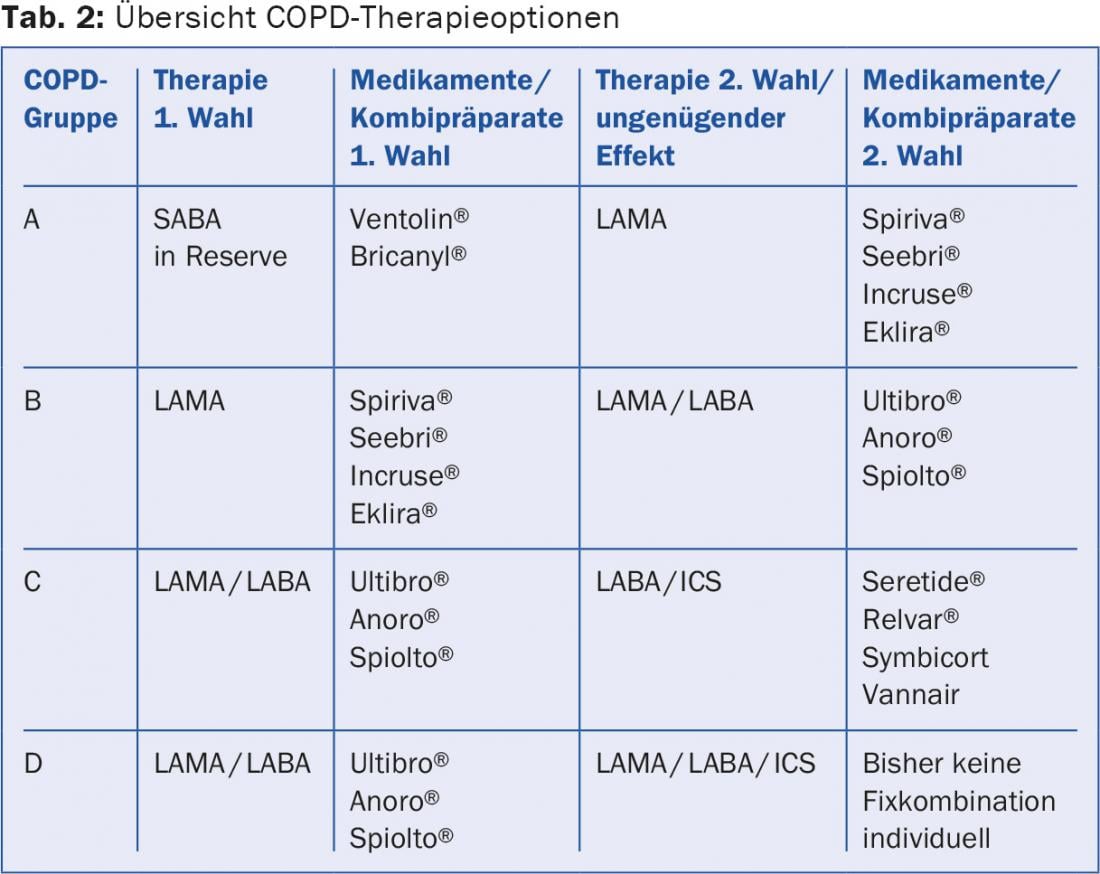
All symptomatic patients should receive a short-acting betamimetic (SABA) as an emergency medication. For group A patients, this is sufficient. More symptomatic patients with moderate obstruction and without frequent exacerbations (group B) receive a LAMA as first choice. If the effect is insufficient or the patient is markedly symptomatic, a combination drug LAMA/LABA is used directly. Multiple studies have shown that the combination of LAMA/LABA is superior to monotherapy and combination therapy LABA/ICS in terms of improvement in lung function, exercise capacity, and quality of life [11].
The importance of ICS has clearly declined in recent years. In any case, their use is only under discussion in case of FEV1 <50 (-60)% of the target and frequent exacerbations. All large studies with ICS had to report a slightly increased pneumonia rate as a negative effect. In group C and D patients, reducing the rate of exacerbations is an important therapeutic goal. The LABA/ICS combination is able to reduce the exacerbation rate at the cost of slightly more frequent pneumonia [12]. But not only steroids, also LAMA and LABA have a beneficial effect on the number of exacerbations. In a head-to-head comparison, the combination LAMA/LABA (Ultibro®) was shown to be not only equivalent but superior to the combination LABA/ICS (Seretide®) in terms of exacerbation rate [13]. Thus, a combination LAMA/LABA is recommended as the first choice for both groups 3 and 4. ICS is indicated when there are clear aspects of additional bronchial asthma (high degree reversibility of obstruction, history of asthma, eosinophilia), a so-called “asthma COPD overlap syndrome” (ACOS) in addition to COPD, or when a bronchodilator is intolerant. It is also possible that patients with mildly increased blood eosinophils (>200 or 300/ μl) may benefit from ICS. In severely symptomatic group D patients, triple therapy LAMA/LABA/ICS may be considered, in addition, of course, to non-inhaled therapeutic measures. Direct comparisons within the substance classes are largely lacking, so that no clear preferences can be formulated.
Bronchial asthma: In contrast to the therapy of COPD, the treatment of bronchial asthma has not changed significantly in recent years. The goals of therapy are optimal symptom control, lung function that is as normal as possible, and prevention of exacerbations [14,15]. A questionnaire such as the “Asthma-Control Test” (ACT) [16,17] has proven useful for recording symptom control.
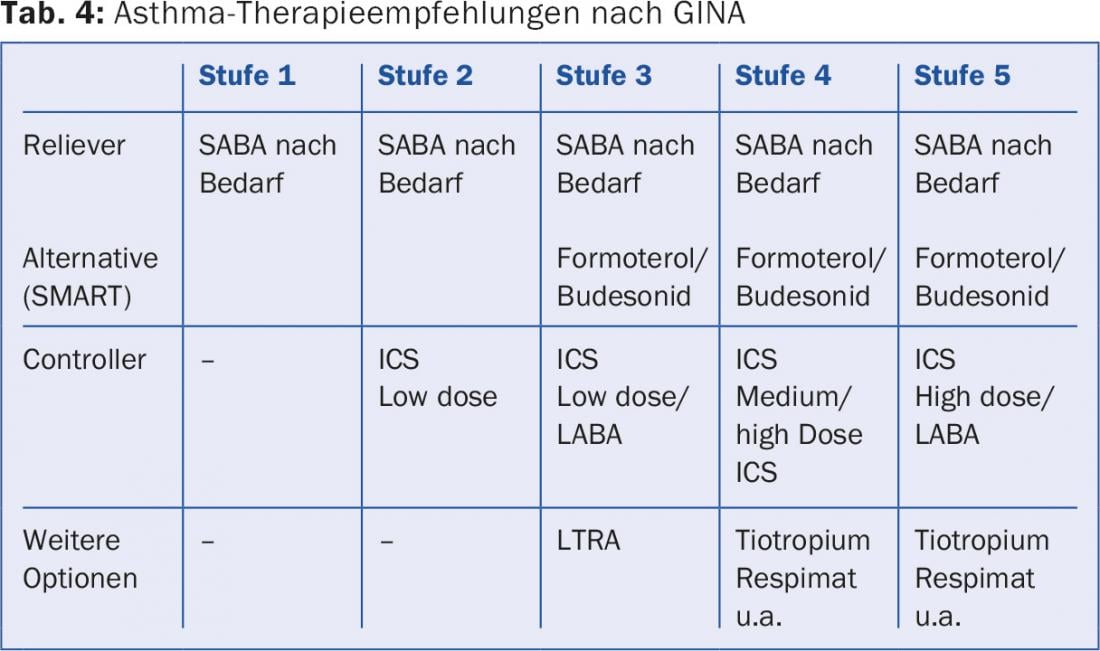
Every asthmatic receives a SABA as an emergency medication (“reliever”). If this is not needed more frequently than 2×/week and the therapy goals are achieved with it (GINA level 1), no further medication is necessary. If this is not the case, the controller is initially ICS in low dosage (GINA level 2) or, in the case of pronounced symptoms, as a combination ICS/LABA (GINA level 3) with dose increase of ICS in case of insufficient effect (GINA level 4). Tactically, it is usually more skillful to start with intensive therapy to quickly achieve good asthma control and then reduce the intensity of therapy: first reduce the dose of ICS and then omit the LABA (“step-up/step-down”). An alternative prescribing practice from stage 3 is the so-called SMART principle (“Symbicort Maintenance and Reliever Therapy”), which is only possible with a LABA with a rapid onset of action as in Symbicort® (formoterol/budesonide). A relatively low base dose (Symbicort TH 200/6 2×1) is prescribed, with instructions to inhale additional Symbicort as needed. Thus, the ICS dose is automatically adjusted to the current need, and identical asthma control is possible with a mostly moderate steroid dose. The new combination preparation Relvar® (fluticasone furoate/vilanterol) allows single dosing. Spiriva®, previously evaluated only for COPD therapy, applied with Respimat®, can be added as another inhaled option (off label) if asthma control under LABA/ICS is insufficient [18] (Tab. 4).
Literature:
- Braido F, et al: “Trying, But Failing” – The Role of Inhaler Technique and Mode of Delivery in Respiratory Medication Adherence. J Allerg Clin Immunol Pract 2016; 4: 823-832.
- Laube BL, et al: What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011; 37: 1308-1331.
- Rothe T: Inhaled therapy – Part 1: Physics and systems. Switzerland Med Forum 2014; 14: 402-406.
- Crompton GK, et al: The need to improve inhalation technique in Europe: a report by the Aerosol Drug Management Improvement Team. Respir Med 2006; 100: 1479-1494.
- Sanchis J, et al: Systematic Review of Errors in Inhaler Use. Has Patient Technique Improved Over Time? CHEST 2016; 150(2): 394-406.
- Haidl P, et al: Inhalation devices requirements for patient’s inhalation maneuvers. Respiratory Medicine 2016; 118: 65-75.
- Soler-Cataluna JJ, et al: Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60: 925-931.
- Jones PW: COPD assessment test – rationale, development, validation and performance. J COPD 2013; 10(2): 269-271.
- www.catestonline.org
- www.goldcopd.org
- Vogelmeier CF, et al: Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med 2013; 1: 51-60.
- Calverley PMA, et al: Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. N Engl J Med 2007; 356: 775-789.
- Wedzicha JA, et al: Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016; 374: 2222-2234.
- www.ginasthma.org
- Reddel HK, et al: A summary of the nex GINA startegy: a roadmap to asthma control. Eur Respi J 2015; 46: 622-639.
- Schatz M, et al: Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006; 117: 549-556.
- www.asthmacontroltest.com/Europe/Switzerland/German
- Rodrigo GJ, et al: What is the role of tiotropium in asthma?: a systematic review with meta-analysis. Chest 2015; 147: 388-396.
HAUSARZT PRAXIS 2016; 11(11): 24-30











