For 1-3 brain metastases, stereotactic radiotherapy is highly effective, either as a single time treatment for smaller lesions or as fractionated stereotactic radiotherapy. In single-treatment radiotherapy, the radiation dose must either be reduced or replaced by fractionated stereotactic radiotherapy, depending on the metastasis size. Compared with stereotactic radiotherapy or surgery alone, additional whole-brain irradiation significantly improves local control of brain metastases and prevents new ones. However, no effect on survival has been demonstrated to date. Therefore, the procedure in these situations must be discussed in detail with the patient and adapted to his or her needs. Palliative whole brain irradiation is now predominantly performed in patients with more than three brain metastases. It has a good palliative effect, but neurocognition impairment must be expected in the long term. Sparing the hippocampal system may prevent deterioration of neurocognitive function.
Approximately 20-30% of all tumor patients develop brain metastases in the course of their tumor disease. In men, the most common primary tumor is lung carcinoma, followed by renal cell carcinoma and melanoma [1]. In women, the most common primary tumor is breast carcinoma, followed by lung carcinoma and melanoma. Brain metastases may occur early in a tumor patient’s medical history, causing the first symptoms of tumor disease. However, brain metastases usually occur in advanced tumor disease.
Due to the complaints, especially the intracranial pressure symptoms with nausea, vomiting and the consecutive reduction of the general condition, the patients’ quality of life is limited. Without therapy, patients usually die within one to three months. Treatment is often difficult because many patients have already received a range of therapies at this point, such as chemotherapy, radiation therapy, and surgery, so patients’ overall treatment reserves are limited. Conversely, in some patients with singular brain metastasis, adequate therapy can still achieve long-term survival, so treatment should be optimal.
Radiation therapy is an essential component of the treatment of brain metastases. This review outlines what options radiotherapy for brain metastases has to offer.
Whole brain irradiation
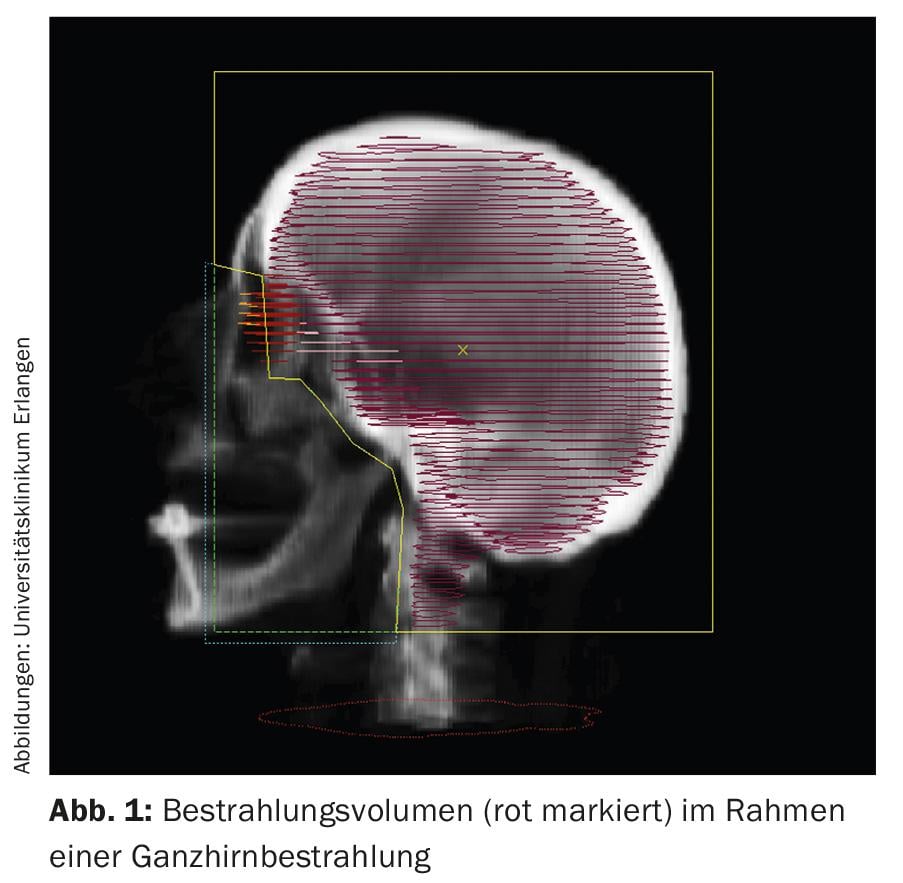
Whole-brain irradiation covers the cerebrum, the cerebellum, and all CSF spaces and meninges in the irradiation fields (Fig. 1) . Whole-brain irradiation can improve neurological symptoms and can also be expected to improve the prognosis for patients.
Whole brain irradiation as prophylactic radiation (to prevent new brain metastases) has gained acceptance in small cell lung cancer (SCLC). In both limited-disease and extensive-disease stages, prophylactic whole-brain irradiation after chemotherapy or radiochemotherapy was shown to decrease the rate of brain metastases from 40% to less than 20% [2]. In SCLC, this led to an improvement in the prognosis of patients at both stages. Therefore, this treatment is considered standard in SCLC. In other tumor entities, such as non-small cell lung cancer (NSCLC), prophylactic whole brain irradiation has not been successful because it has reduced brain metastasis but has not improved survival.
Whole-brain irradiation has been increasingly criticized in recent years because of the increased incidence of neurocognitive effects such as reduction in cognitive function, fatigue, loss of appetite, and reduction in general condition after whole-brain irradiation [3,4]. Also, several studies showed that no effect on patient survival prognosis can be achieved by whole-brain irradiation compared with stereotactic irradiation alone for up to three brain metastases. However, it must be considered that the rate of new-onset brain metastases was significantly reduced by whole-brain irradiation. This is true for the area of stereotactically irradiated regions as well as for the area of new brain metastases (Tab. 1-3).
Protection of the hippocampus
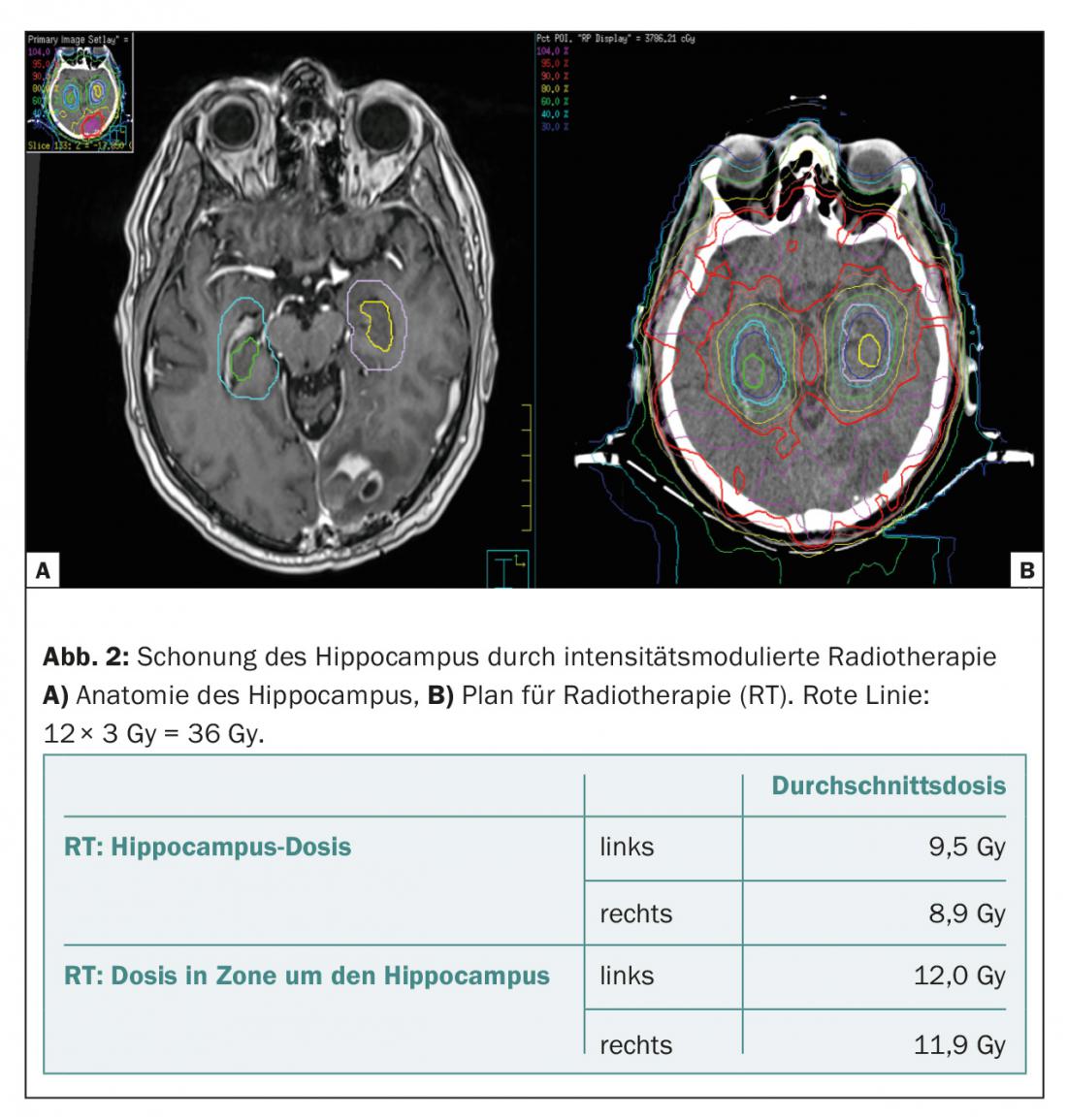
One way to reduce the cognitive side effects of whole-brain irradiation has been investigated in recent years in terms of dose reduction in the hippocampal region by intensity-modulated irradiation [5]. It is well known that hippocampal loss can result in severe antegrade loss of memory function, spatial orientation, and emotional ability [6]. The hippocampus is very sensitive to irradiation, as the dose that can lead to these side effects is as low as 10-20 Gy [7]. Because very few brain metastases manifest in the hippocampal region (0.3-2.3%) [5,8–10], the first randomized trials comparing whole-brain irradiation with and without hippocampal sparing are now underway. Gondi et al. reported in a single-arm Phase II study (RTOG 0933) that 113 patients treated with a dose of 10× 3 Gy, neurocognitive function (as measured by Hopkins Verbal Learning Test and Revised Delayed Recall Test) deteriorated significantly less when the hippocampus was spared (compared to historical controls). (Fig. 2).
Stereotactic irradiation
Stereotactic irradiation is the irradiation of a relatively small tumor volume, which is well demarcated against adjacent healthy tissue, with a higher irradiation dose (4-22 Gy). Radiosurgery, in which a single stereotactic irradiation with a high dose (13-22 Gy) is applied, is distinguished from fractionated stereotactic irradiation, in which multiple stereotactic irradiations with a reduced dose, e.g., 4× 10 Gy to 12× 4 Gy, are applied.
In the early years, patients were accurately positioned using a bloody fixation system. We have moved away from that nowadays. Nowadays, patients receive a plastic mask, bite blocks, etc. as standard for positioning. For stereotaxy, the “Gammaknife” system was mainly used in the earlier years; nowadays, dedicated linear accelerators, such as the Novalis accelerator system, or a Cyberknife device are also available. Stereotaxy can also be performed with most commercially available accelerators.
Advantages and disadvantages of radiosurgery
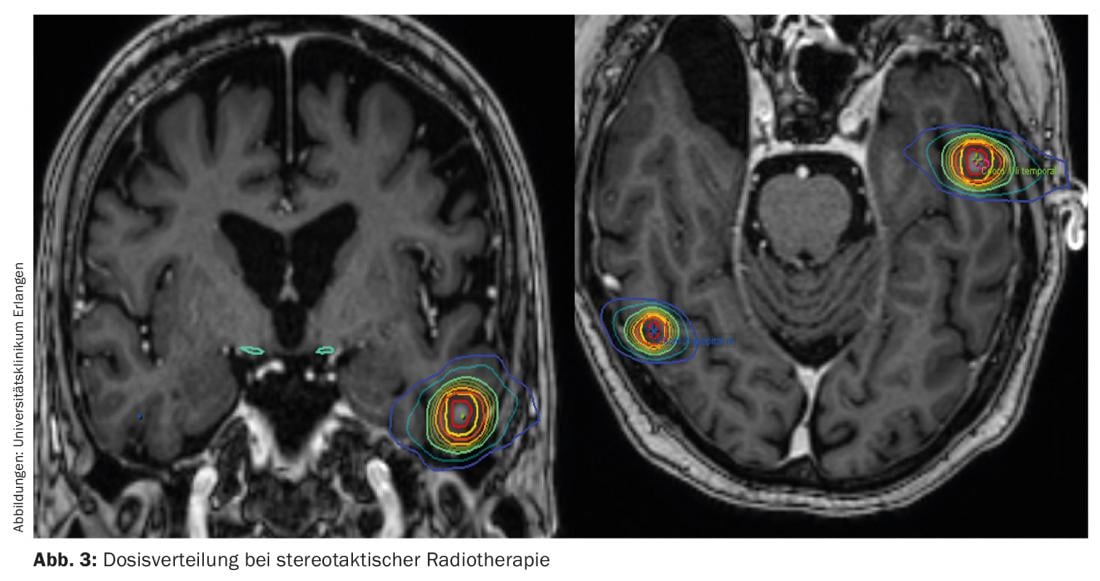
Advantages of radiosurgery include, in particular, the minimally to noninvasive approach. Due to the precisely defined target volume to the millimeter and the steep dose edge drop, maximum sparing of the surrounding healthy tissue is achieved (Fig. 3). Another advantage of stereotactic irradiation is that it can be performed multiple times, especially when patients develop brain metastases in new localizations [12]. However, a disadvantage compared to surgery is that no histological confirmation of the diagnosis can be made.
Stereotactic irradiation in terms of radiosurgery was initially performed in the presence of a metastasis up to 1-2 cm in diameter. Thus, after application of 20-22 Gy, local control rates of 70-90% could be achieved. Nowadays, up to three brain metastases are also regularly treated, either with single time stereotactic radiotherapy for diameters <2 cm or fractionated stereotactic radiotherapy for larger diameters. Stereotactic radiotherapy alone of 5-10 brain metastases may also be quite effective [13].
The side effects of radiosurgery acutely include symptomatic edema (7-10%), nausea, vomiting, and, very rarely, convulsions. In the long term, radiation therapy may occasionally cause intralesional bleeding. Radionecrosis is much more common, and it is often very difficult to distinguish it from tumor progression. Kohutec et al. found radionecrosis in up to 25% of patients, of whom 17% became clinically symptomatic, i.e., suffered from increasing brain edema. The tumor size is mainly responsible for radionecrosis. For tumor metastases less than 0.5 cm, radionecrosis occurred in less than 10% of cases; for tumor size greater than 1.5 cm, it occurred in up to 60%. In addition, there is the applied dose. A dose of 20-22 Gy is now considered well tolerated. However, for larger brain metastases, the dose must be reduced, and thus local control may also deteriorate. The Radiation Therapy Oncology Group (RTOG) recommends a dose of 20-22 Gy for brain metastases up to 2 cm, 18 Gy for tumors 2-3 cm, and 15 Gy for tumors larger than 3 cm.
Due to the limited dose in larger tumors, more and more research groups are moving towards fractionated stereotactic irradiation rather than single time irradiation for these tumors. The irradiation doses applied in this process are 10× 4 Gy, 7× 5 Gy, 6× 6 Gy, etc. In a retrospective analysis, fractionated stereotactic irradiation was shown to significantly decrease the necrosis rate (from 17% to 5%; p <0.05) compared to single time irradiation, while maintaining local control (69% versus 71%) [15]. Corresponding results are also reported from other beam groups [16].
Single time irradiation alone or whole brain plus single time irradiation?
The additional application of stereotactic irradiation (15-24 Gy) to whole brain irradiation (2.5-37.5 Gy) improves the prognosis of patients with singular brain metastases and smaller brain metastases: Median survival increased from 4.9 to 6.5 months in one study (p=0.039) [17]. In addition, the additional stereotactic irradiation applied to these patients significantly improved their general condition and significantly reduced the consumption of steroids.
A whole series of randomized trials has now investigated whether the therapeutic results of stereotactic irradiation or microsurgical resection can be improved by additional whole-brain irradiation (Table 1-3) [18–21]. In all studies, additional whole-brain irradiation was shown to improve local control of preexisting brain metastases, from 67-90% to 84-100%. In addition, whole brain irradiation significantly reduced the incidence of new brain metastases: After stereotactic irradiation alone, new brain metastases occurred in 32-63%, after additional whole-brain irradiation only in 10-50%. However, none of the studies showed that the prognosis of the patients would have improved. The prognosis of the patients was almost identical in the studies; in Chang et al. the prognosis was even reduced with additional whole-brain irradiation.
Recently, results from the Phase III NCCTG N0574 (Alliance) trial were presented at the 2015 ASCO Congress [22]. This work, which has not yet been published as a full manuscript, also shows an improvement in intracranial control with the addition of whole-brain irradiation, but no improvement in overall survival, in agreement with the four papers mentioned above.
In a recent analysis from Japan, Aoyama et al. In contrast, show that in certain patient collectives with a good prognosis (DS-GPA score 2.5-4.0), additional whole-brain irradiation can improve overall survival (2-year survival without whole-brain irradiation 20%, with whole-brain irradiation 50%, p=0.04) [23].
Because of the rate of side effects in the cognitive area, very many research groups have moved away from whole-brain irradiation for 1-3 brain metastases, and instead only stereotactic irradiation of the individual brain metastases. In contrast, we prefer a staged approach depending on histology and number of metastases:
- In patients with singular brain metastasis, stereotactic radiation is mostly performed, except in SCLC.
- In the case of 2-3 brain metastases, stereotactic radiation alone is also used for malignant melanoma, colon carcinoma, and renal cell carcinoma. For patients with NSCLC or breast carcinoma, detailed patient education is provided so that patients can then decide whether they want stereotactic radiotherapy alone or stereotactic radiotherapy plus whole-brain irradiation.
Postoperative irradiation
Older studies have shown that postoperative whole brain irradiation can improve the prognosis of patients after surgical removal of a brain metastasis [24–26]. In particular, intracranial control of brain metastases was significantly improved in these patients. This effect was also shown in the EORTC study 22952-26001 [27]. After surgery alone, brain metastases recurred in 60% of patients; additional whole-brain irradiation halved this rate to 30%. Despite this, the prognosis of patients in the EORTC trial did not improve. Therefore, postoperative whole-brain irradiation has been increasingly questioned.
Alternatively, local radiotherapy of the resection cavity is increasingly preferred. This can also achieve very good local control rates of up to 90% [28]. Therefore, for singular metastases, we usually perform local stereotactic radiotherapy; for multiple foci, patients are informed about the risks and opportunities of whole-brain irradiation and are included in the decision-making process.
Recurrences of brain metastases
Stereotactic radiation may also be effective for brain metastases that recur after whole-brain irradiation. In brain metastases that recur after whole-brain irradiation, a study showed that stereotactic single-time irradiation with 20-22 Gy can achieve local control of another 50-60% [29]. However, patients must be informed of the increased rate of side effects.
It is unclear whether whole-brain irradiation is still effective after multiple stereotactic irradiations. There is little reliable data available here. We apply another 12-20× 2 Gy along with chemotherapy in these situations. Patients must also be informed about increased side effects.
Bibliography by the authors
InFo ONCOLOGY & HEMATOLOGY 2016; 4(4):16-20.