Various studies have recently led to an expansion of indications for cardiac resynchronization therapy. The desire to avoid intravascular leads connected to a subcutaneous device has led to the development of subcutaneously implantable ICDs and catheter-delivered, probeless pacemakers. While the former are already used in clinical practice after careful patient selection, the latter are only used in studies. Efforts to develop a complete two- or three-chamber system without electrical conductors are underway. Infection complications after cardiac device implantation and replacement are a dreaded problem. Antibiotic-coated device wraps are one promising approach to reducing infection rates. However, further studies are needed on this and on systemic periinterventional antibiotic administration.
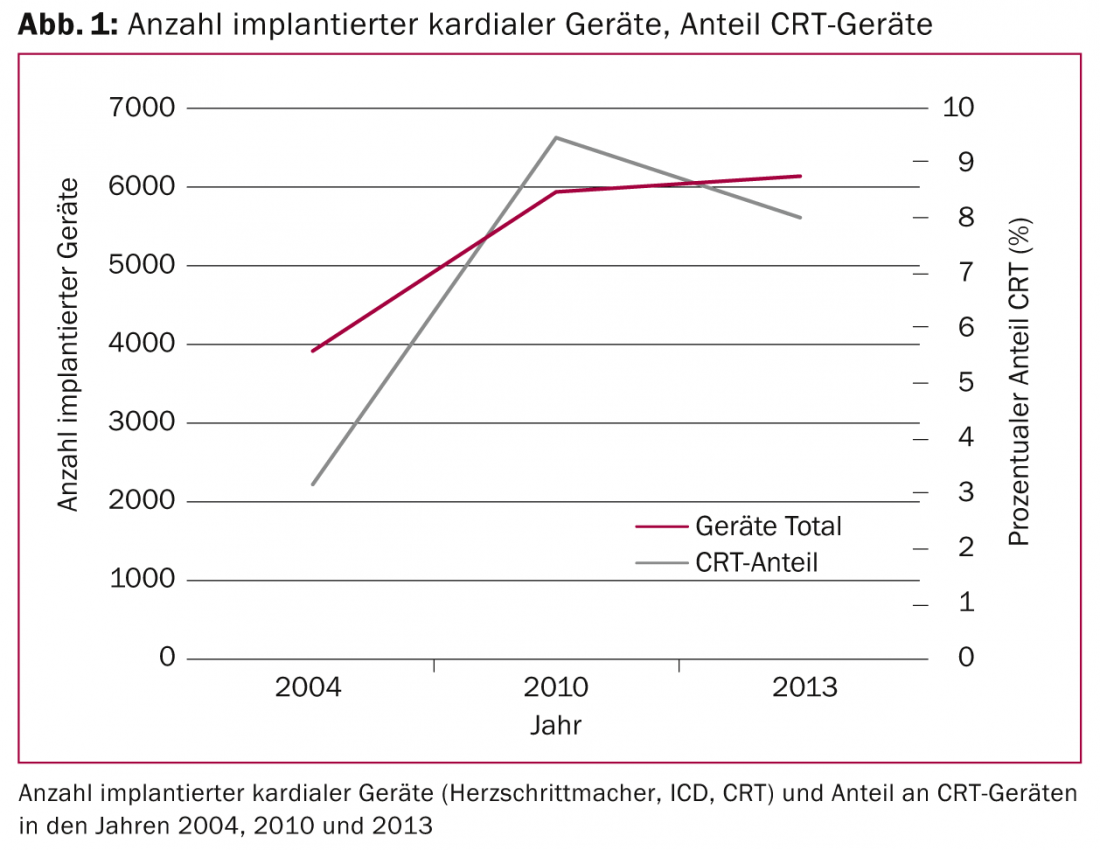
The indications for implantation of cardiac devices (conventional pacemakers, implantable defibrillators [ICD] and biventricular pacing systems) have expanded over the past decade due to new discoveries, especially in the field of cardiac resynchronization therapy (CRT). While just under 4000 cardiac devices were implanted in Switzerland in 2004, this figure grew to a good 6000 devices in 2013. The share of CRT devices tripled between 2004 and 2010 (Fig. 1) .
A technical milestone was the introduction of the subcutaneous ICD and the demonstration of the feasibility/safety of leadless pacing. However, while these new technologies are only gradually finding their way into routine clinical practice, the performance of MR examinations in patients with non-MR-compatible devices or the avoidance of device infections are common challenges in everyday clinical practice that will be briefly addressed in this review article.
Patient selection for cardiac resynchronization therapy.
In the field of CRT, numerous interesting studies have been performed and published in recent years. The CARE-HF extension study demonstrated a significant relative all-cause mortality risk reduction of 40% (absolute reduction from 38.1 to 24.7%) within 3 years with CRT in patients with optimally treated severe heart failure (NYHA III/IV), LVEF ≤35%, and a widened QRS complex. In patients with milder heart failure (NYHA I/II) and a primary prophylactic ICD indication, additional CRT in the REVERSE trial did not reduce the primary composite endpoint, but it did reduce heart failure-related hospitalizations and favorably affect left ventricular geometry with significant increases in ejection fraction. Annual mortality in the recently published 5-year follow-up was surprisingly low at 2.9%, which at least partially explains the lack of effect of therapy on this endpoint in 610 patients included . The similar RAFT trial compared CRT-ICD therapy with ICD therapy alone and found a significant reduction in the primary endpoint composed of all-cause mortality and heart failure-related hospitalization in patients with NYHA II/III heart failure. Taken together, these two papers suggest that patients with milder heart failure also benefit from CRT. Additional expansion of CRT may also result in the future from the positive results of the recently published block HF trial, in which patients with a pacing indication for slow arrhythmia benefited from CRT over conventional pacemaker therapy regardless of their NYHA class if left ventricular pump function was less than 50%.
The fact that patients with a QRS width <120 ms may have echocardiographic dyssynchrony and that some monocentric studies demonstrated a benefit in heart failure symptoms from CRT in these patients led to the idea of extending the concept of CRT to patients with a relatively narrow QRS. However, the two multicenter randomized controlled trials LESSER-EARTH and EchoCRT showed not only a lack of survival benefit of CRT compared with ICD therapy alone, but even excess mortality in the CRT group (EchoCRT), so that CRT is now a contraindication in patients with a QRS <120 ms without pacing indication.
The downside of increasing CRT indications is certainly the higher complication rate of CRT devices compared with ICD devices. In a multivariable analysis, CRT-D implantation was associated with a 2.2-fold increased risk of complications within 45 days of implantation compared with single-chamber ICD implantation [1], and CRT-D implantation is an independent risk factor for device revisions compared with ICD implantation alone.
Multisite LV pacing
Decreased response to CRT may be observed with increasing LV scar fraction, posterolateral scar, or extreme mechanical dyssynchrony [2]. Scar areas have unfavorable electrical conduction properties, and pacing in a myocardial scar area is therefore undesirable. The TARGET and STARTER trials demonstrate that targeted LV probe placement in echocardiographically identified late-excited myocardial segments can favorably influence prognosis [2]. Multipolar LV probes with multiple stimulation vectors to choose from may offer advantages in this regard, as viable myocardial segments and/or late excited areas can be specifically stimulated even after implantation of the (multipolar) LV probe by appropriate programming. Furthermore, possible phrenic stimulation can be bypassed by changing the stimulation vector. The theoretical advantages are convincing and it remains to be seen whether the few available data confirming this concept will be consolidated in larger studies.
can.
Subcutaneous ICD
The transvenous ICD systems developed in the 1980s require complexly constructed electrodes that must perform both sensing/pacing and defibrillator functions. It is therefore not surprising that, despite innovative technologies, these electrodes are prone to defects, as Medtronic SprintFidelis or SJM Riata electrodes have shown us. In a monocentric German study of 990 ICD patients, 15% had electrode problems during follow-up (median 2.5 years) [3], 56 insulation defects, 12 electrode fractures, and 31% had other problems such as “loss of capture,” sensing, or impedance problems. Although these high numbers could not be confirmed in a multicenter study in which the 5-year rate of electrode revisions was 2.5% [4], there can be no doubt that the greatest weakness of an ICD is the electrode. It is therefore tempting to offer antitachycardia protection without the difficulties associated with transvenous leads; this approach became at least partially possible with the introduction of the S-ICD, which does not require an intravenous lead (Fig. 2) .
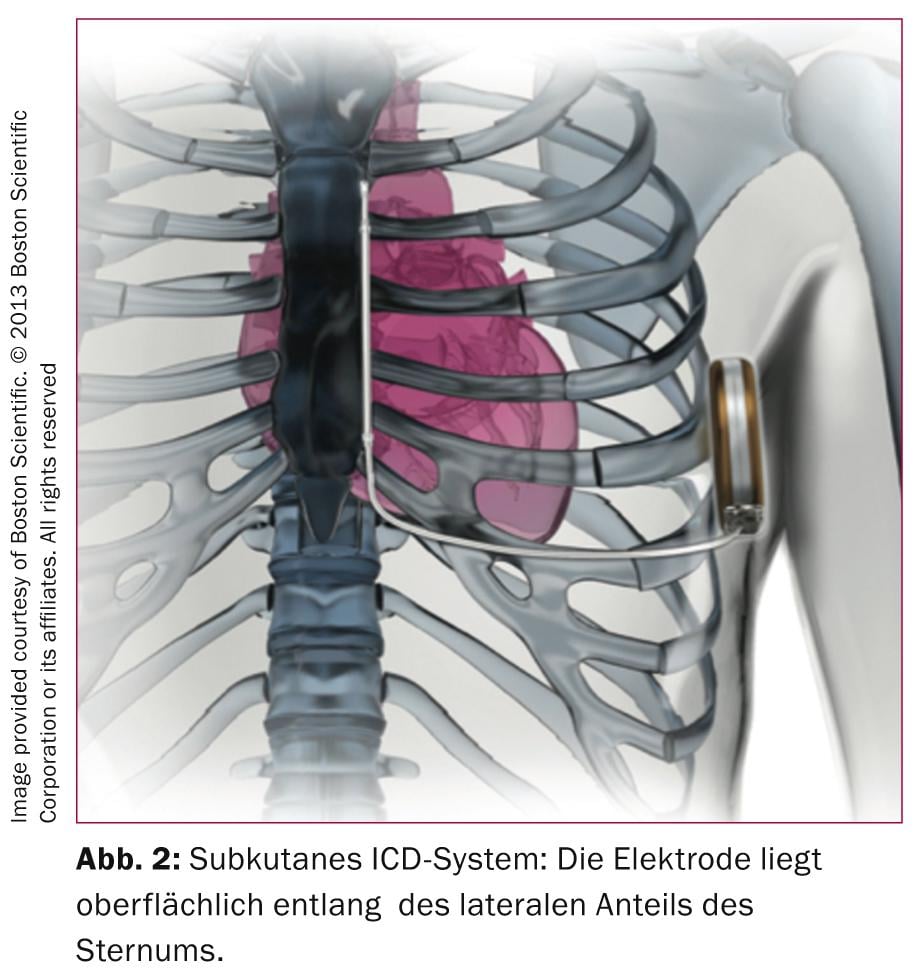
The absence of an intravascular electrode has advantages during device implantation (reduction of X-ray radiation dose) and it prevents future electrode problems with possible need for complicated electrode extractions. The disadvantage of the S-ICD is that in the absence of intracardiac electrography leads, detection of ventricular fibrillation may be difficult, which is why the surface ECG must be analyzed in detail before implantation of an S-ICD. However, according to the most comprehensive registry data published to date [5], the rate of inappropriate shocks is 7%, i.e., compared with conventional ICD systems, the rate of unwarranted ICD shocks is rather low [6]. Antitachypacing to overstimulate ventricular tachycardia is not possible with subcutaneous systems (or would not be tolerated by patients), which is why these systems tend to be used less in patients with coronary artery disease or nonischemic cardiomyopathy and the main indications are patients with ion channel defects, hypertrophic cardiomyopathy, or patients after ICD infection with transvenous systems. Finally, a stronger current surge by charging the capacitor to 80 J is required to terminate ventricular tachycardia/fibrillation, which requires a larger device. Thus, in individual cases, a critical weighing of the advantages and disadvantages is necessary before implanting an S-ICD.
Leadless pacing
Many complications of device implantation are due to the fact that electrodes must be advanced and anchored intravascularly in the heart and are subjected to constant mechanical stress throughout life. Therefore, a probe-less way to stimulate the heart is very attractive. In the LEADLESS study, the Nanostim™ Leadless Pacemaker System developed by Nanostim (acquired by St. Jude Medical) was first tested in 33 patients and found to be feasible and safe. Medtronic has developed a similar system (Micra™ Transcatheter Pacing System), which can also be anchored to the apex of the right ventricle via a steerable catheter from the groin. The battery of this system has a predicted service life of approximately eight years. Technical efforts to enable communication between various small pacemakers placed in right atrium and both ventricles, paving the way for leadless two- and three-chamber systems, are underway. Should these systems come into widespread use in the future , as we are convinced they will, it will greatly change our implantation technique, as such systems must be inserted from inguinal in an electrophysiology/cardiac catheterization laboratory, and current catheters not yet on the market today are between 18 and 23 F in diameter. In inexperienced hands, this inevitably leads to complications and poses new challenges for hospital logistics.
Magnetic resonance imaging in patients with cardiac devices.
It is estimated that up to 75% of patients with a pacemaker will have an indication for an MR scan during their lifetime. This realization is taken into account by an increasing number of MR-compatible devices offered by different manufacturers, which are often and gladly implanted in everyday life. Despite the growing popularity of these devices, the problem of a desired MR examination in patients with existing non-MR capable systems often arises in clinical practice. In these cases, an MRI examination is usually not feasible. Although evidence is mounting that MR examinations are relatively low in complications even in these patients, all ICDs and most pacemakers are considered contraindications to MR examination by the U.S. FDA as well as by device manufacturers. Nevertheless, according to the latest ESC guidelines [7], after exclusion of patients with freshly implanted devices or left non-connected electrode (fragments), an MR examination is justifiable in case of absolute necessity (no alternative slice imaging technique) after careful benefit/risk assessment even in patients without MR-suitable devices and can be performed with 1.5 Tesla at low risk of complications, provided adequate preparation has been made.
Reduction of the risk of infection during device implantation
Infection is probably the most feared complication of device implantation and is associated with high economic costs and also increased mortality. Risk factors for device infections include re-intervention, fever, and/or lack of antibiotic infection prophylaxis [8]. Interestingly, up to one-third of ICD lodges or electrodes are bacterially colonized at ICD replacement [9], which may be related to the increased risk of infection after re-interventions. The only randomized controlled trial comparing periinterventional antibiotic administration vs placebo before pacemaker implantation had to be stopped prematurely after recruitment of two thirds of the planned patients because of the clear advantage of antibiotic administration [10]. In a recently published prospective registry study, additional postoperative antibiotic administration for four days was shown to result in a further significant reduction in device infections in both initial surgery and reoperations [11]. Furthermore, few but promising data are available for a recently available absorbable antibiotic mesh (Fig. 3) [12].

This mesh, self-absorbed after nine weeks and coated with minocycline and rifampicin , significantly reduced the infection rate from 3 to 0.9% in a selected patient population compared with a historical control and is particularly effective in patients at high risk of infection [12]. Further studies are necessary and in progress (CITADEL/CENTURION study).
Outlook
The technical innovations show the increasing effort to move away from transcutaneously inserted electrodes, be it subcutaneous ICD or probeless pacing. Since large implantation numbers are still lacking and endpoint data must first confirm the promising preliminary results, it remains to be seen whether and how quickly these new technologies will become established in clinical practice. Regarding reduction of infection rate, especially after device change of tachydevices, we urgently need new studies investigating peri- and postoperative antibiotic administration. Retrospective evidence for prolonged antibiosis has been published, and the positive data from antibiotic-soaked wraps support the concept of prolonged antibiosis for infection prevention.
Dr. med. Dr. scient. med. Roman Brenner
David Altmann, MD
Prof. Dr. med. Peter Ammann
Literature:
- Lee DS, et al: Evaluation of early complications related to De Novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol 2010; 55(8): 774-782.
- Leyva F, Nisam S, Auricchio A: 20 years of cardiac resynchronization therapy. J Am Coll Cardiol 2014; 64(10): 1047-1058.
- Kleemann T, et al: Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation 2007; 115(19): 2474-2480.
- Eckstein J, et al: Necessity for surgical revision of defibrillator leads implanted long-term: causes and management. Circulation 2008; 117(21): 2727-2733.
- Lambiase PD, et al: Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J 2014; 35(25): 1657-1665.
- Poole JE, Gold MR: Who should receive the subcutaneous implanted defibrillator: The subcutaneous implantable cardioverter defibrillator (ICD) should be considered in all ICD patients who do not require pacing. Circ Arrhythm Electrophysiol 2013; 6(6): 1236-1244; discussion 1244-1245.
- Brignole M, et al.: 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013; 34(29): 2281-2329.
- Klug D, et al: Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116(12): 1349-1355.
- Kleemann T, et al: Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace 2010; 12(1): 58-63.
- De Oliveira JC, et al: Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009; 2(1): 29-34.
- Senaratne JM, et al: A 19-year study on pacemaker-related infections: a claim for using postoperative antibiotics. Pacing Clin Electrophysiol 2014; 37(8): 947-954.
- Mittal S, et al: Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014; 11(4): 595-601.
CARDIOVASC 2015; 14(1): 17-20